当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Valve Type and Anesthesia Strategy for TAVR: 5-Year Results of the SOLVE-TAVI Trial
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.jacc.2024.09.007 Hans-Josef Feistritzer, Thomas Kurz, Reinhard Vonthein, Leonie Schröder, Georg Stachel, Ingo Eitel, Christoph Marquetand, Roza Saraei, Eva Kirchhof, Matthias Heringlake, Mohamed Abdel-Wahab, Steffen Desch, Holger Thiele
中文翻译:

瓣膜类型和麻醉策略对 TAVR 的影响:SOLVE-TAVI 试验的 5 年结果
在随机 SOLVE-TAVI (第二代 seLf 扩张瓣膜与球囊扩张瓣膜和经导管主动脉瓣植入术中血管与局部麻醉的比较)试验中,比较了新一代自扩张瓣膜 (SEV) 和球囊扩张瓣膜 (BEV),以及清醒镇静 (CS) 和全身麻醉 (GA),瓣膜和麻醉比较在 30 天和 1 年的临床结局相似。假体的耐用性可能会影响长期随访期间的临床结局。此外,到目前为止,麻醉策略对长期临床结果的影响尚不清楚。
作者试图比较随机 SOLVE-TAVI 试验中 5 年随访期间的临床结果。
在随机、多中心、2 × 2 析因、开放标签 SOLVE-TAVI 试验中,447 名患有严重症状性主动脉瓣狭窄的中高风险患者被随机分配到经股经导管主动脉瓣置换术 (TAVR) 组,使用 SEV (Evolut R,美敦力) 或 BEV (SAPIEN 3,Edwards Lifesciences) 以及 CS 与 GA。患者随访 5 年。
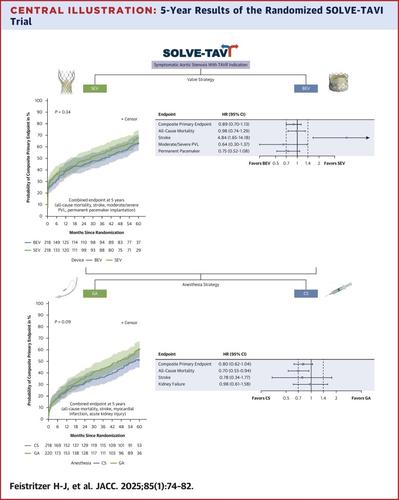
在 5 年的随访中,SEV 和 BEV 组的全因死亡率、卒中、中度或重度瓣周渗漏和永久性起搏器植入的联合预定终点相似 (67.7% vs 63.4%;心率:0.89;95% CI:0.70-1.13;P = 0.34)。SEV 组 5 年卒中发生率较低 (2.2% vs 9.6%;心率:4.84;95% CI:1.65-14.18;P = 0.002)。关于麻醉比较,全因死亡率、卒中、心肌梗死和急性肾损伤的主要终点发生在 CS 组 51.4% 和 GA 组 61.3% (HR: 0.80;95% CI: 0.62-1.04;P = 0.09)。CS 的 5 年全因死亡率较低 (41.5% vs 54.3%;心率:0.70;95% CI:0.53-0.94;P = 0.02)。
使用联合临床终点,使用 SEV 和 BEV 以及 CS 和 GA 的经股动脉 TAVR 在 5 年时显示出相似的临床结果。
更新日期:2024-11-06
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.jacc.2024.09.007 Hans-Josef Feistritzer, Thomas Kurz, Reinhard Vonthein, Leonie Schröder, Georg Stachel, Ingo Eitel, Christoph Marquetand, Roza Saraei, Eva Kirchhof, Matthias Heringlake, Mohamed Abdel-Wahab, Steffen Desch, Holger Thiele
|
Background
In the randomized SOLVE-TAVI (compariSon of secOnd-generation seLf-expandable vs. balloon-expandable Valves and gEneral vs. local anesthesia in Transcatheter Aortic Valve Implantation) trial comparing newer-generation self-expanding valves (SEV) and balloon-expandable valves (BEV), as well as conscious sedation (CS) and general anesthesia (GA), clinical outcomes were similar both for valve and anesthesia comparison at 30 days and 1 year. Prosthesis durability may affect clinical outcomes during long-term follow-up. Moreover, the impact of the anesthesia strategy on long-term clinical outcomes is unknown so far.Objectives
The authors sought to compare clinical outcomes during 5-year follow-up in the randomized SOLVE-TAVI trial.Methods
In the randomized, multicenter, 2 × 2 factorial, open-label SOLVE-TAVI trial, 447 intermediate- to high-risk patients with severe, symptomatic aortic stenosis were randomly assigned to transfemoral transcatheter aortic valve replacement (TAVR) using either SEV (Evolut R, Medtronic) or BEV (SAPIEN 3, Edwards Lifesciences) and also to CS vs GA. Patients were followed-up for 5 years.Results
During 5 years of follow-up, the combined predefined endpoint of all-cause mortality, stroke, moderate or severe paravalvular leakage, and permanent pacemaker implantation was similar in the SEV and BEV groups (67.7% vs 63.4%; HR: 0.89; 95% CI: 0.70-1.13; P = 0.34). Stroke rates at 5 years were lower in the SEV group (2.2% vs 9.6%; HR: 4.84; 95% CI: 1.65-14.18; P = 0.002). Regarding the anesthesia comparison, the primary endpoint of all-cause mortality, stroke, myocardial infarction, and acute kidney injury occurred in 51.4% in the CS group and 61.3% in the GA group (HR: 0.80; 95% CI: 0.62-1.04; P = 0.09). All-cause mortality at 5 years was lower for CS (41.5% vs 54.3%; HR: 0.70; 95% CI: 0.53-0.94; P = 0.02).Conclusions
Transfemoral TAVR using either SEV and BEV as well as CS and GA showed similar clinical outcomes at 5 years using a combined clinical endpoint.中文翻译:

瓣膜类型和麻醉策略对 TAVR 的影响:SOLVE-TAVI 试验的 5 年结果
背景
在随机 SOLVE-TAVI (第二代 seLf 扩张瓣膜与球囊扩张瓣膜和经导管主动脉瓣植入术中血管与局部麻醉的比较)试验中,比较了新一代自扩张瓣膜 (SEV) 和球囊扩张瓣膜 (BEV),以及清醒镇静 (CS) 和全身麻醉 (GA),瓣膜和麻醉比较在 30 天和 1 年的临床结局相似。假体的耐用性可能会影响长期随访期间的临床结局。此外,到目前为止,麻醉策略对长期临床结果的影响尚不清楚。
目标
作者试图比较随机 SOLVE-TAVI 试验中 5 年随访期间的临床结果。
方法
在随机、多中心、2 × 2 析因、开放标签 SOLVE-TAVI 试验中,447 名患有严重症状性主动脉瓣狭窄的中高风险患者被随机分配到经股经导管主动脉瓣置换术 (TAVR) 组,使用 SEV (Evolut R,美敦力) 或 BEV (SAPIEN 3,Edwards Lifesciences) 以及 CS 与 GA。患者随访 5 年。
结果
在 5 年的随访中,SEV 和 BEV 组的全因死亡率、卒中、中度或重度瓣周渗漏和永久性起搏器植入的联合预定终点相似 (67.7% vs 63.4%;心率:0.89;95% CI:0.70-1.13;P = 0.34)。SEV 组 5 年卒中发生率较低 (2.2% vs 9.6%;心率:4.84;95% CI:1.65-14.18;P = 0.002)。关于麻醉比较,全因死亡率、卒中、心肌梗死和急性肾损伤的主要终点发生在 CS 组 51.4% 和 GA 组 61.3% (HR: 0.80;95% CI: 0.62-1.04;P = 0.09)。CS 的 5 年全因死亡率较低 (41.5% vs 54.3%;心率:0.70;95% CI:0.53-0.94;P = 0.02)。
结论
使用联合临床终点,使用 SEV 和 BEV 以及 CS 和 GA 的经股动脉 TAVR 在 5 年时显示出相似的临床结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号