Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IspE kinase as an anti-infective target: Role of a hydrophobic pocket in inhibitor binding
Structure ( IF 4.4 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.str.2024.10.009 Rawia Hamid, Danica J. Walsh, Eleonora Diamanti, Diana Aguilar, Antoine Lacour, Mostafa M. Hamed, Anna K.H. Hirsch
Structure ( IF 4.4 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.str.2024.10.009 Rawia Hamid, Danica J. Walsh, Eleonora Diamanti, Diana Aguilar, Antoine Lacour, Mostafa M. Hamed, Anna K.H. Hirsch
|
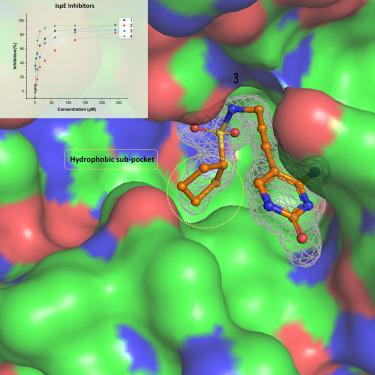
Enzymes of the methylerythritol phosphate (MEP) pathway are potential targets for antimicrobial drug discovery. Here, we focus on 4-diphosphocytidyl-2-C -methyl-D-erythritol (IspE) kinase from the MEP pathway. We use biochemical and structural biology methods to investigate homologs from pathogenic microorganisms; Escherichia coli , Klebsiella pneumoniae , and Acinetobacter baumannii . We determined the X-ray crystal structures of IspE-inhibitor complexes and studied inhibitors’ binding modes targeting the substrate pocket. The experimental results indicate the need for distinct inhibitor strategies due to structural differences among IspE homologs, particularly for A. baumannii IspE, which displays a unique inhibitory profile due to a tighter hydrophobic subpocket in the substrate binding site. This study enhances our understanding of the MEP enzymes and sets the stage for structure-based drug design of selective inhibitors to combat pathogenic microorganisms.
中文翻译:

IspE 激酶作为抗感染靶点:疏水口袋在抑制剂结合中的作用
磷酸甲基赤藓糖醇 (MEP) 途径的酶是抗菌药物发现的潜在靶标。在这里,我们专注于来自 MEP 通路的 4-二磷酸胞苷-2-C-甲基-D-赤藓糖醇 (IspE) 激酶。我们使用生化和结构生物学方法来研究病原微生物的同源物;大肠埃希菌、肺炎克雷伯菌和鲍曼不动杆菌。我们确定了 IspE 抑制剂复合物的 X 射线晶体结构,并研究了靶向底物袋的抑制剂结合模式。实验结果表明,由于 IspE 同源物之间的结构差异,需要不同的抑制剂策略,特别是对于鲍曼曲霉 IspE,由于底物结合位点中更紧密的疏水子袋,它显示出独特的抑制特征。这项研究增强了我们对 MEP 酶的理解,并为基于结构的选择性抑制剂药物设计以对抗病原微生物奠定了基础。
更新日期:2024-11-06
中文翻译:

IspE 激酶作为抗感染靶点:疏水口袋在抑制剂结合中的作用
磷酸甲基赤藓糖醇 (MEP) 途径的酶是抗菌药物发现的潜在靶标。在这里,我们专注于来自 MEP 通路的 4-二磷酸胞苷-2-C-甲基-D-赤藓糖醇 (IspE) 激酶。我们使用生化和结构生物学方法来研究病原微生物的同源物;大肠埃希菌、肺炎克雷伯菌和鲍曼不动杆菌。我们确定了 IspE 抑制剂复合物的 X 射线晶体结构,并研究了靶向底物袋的抑制剂结合模式。实验结果表明,由于 IspE 同源物之间的结构差异,需要不同的抑制剂策略,特别是对于鲍曼曲霉 IspE,由于底物结合位点中更紧密的疏水子袋,它显示出独特的抑制特征。这项研究增强了我们对 MEP 酶的理解,并为基于结构的选择性抑制剂药物设计以对抗病原微生物奠定了基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号