Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Innate immunity-modulating nanobiomaterials for controlling inflammation resolution
Matter ( IF 17.3 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.matt.2024.09.016 Yongjiang Li, Wei Chen, Seyoung Koo, Haijun Liu, Qimanguli Saiding, Angel Xie, Na Kong, Yihai Cao, Reza Abdi, Charles N. Serhan, Wei Tao
Matter ( IF 17.3 ) Pub Date : 2024-11-06 , DOI: 10.1016/j.matt.2024.09.016 Yongjiang Li, Wei Chen, Seyoung Koo, Haijun Liu, Qimanguli Saiding, Angel Xie, Na Kong, Yihai Cao, Reza Abdi, Charles N. Serhan, Wei Tao
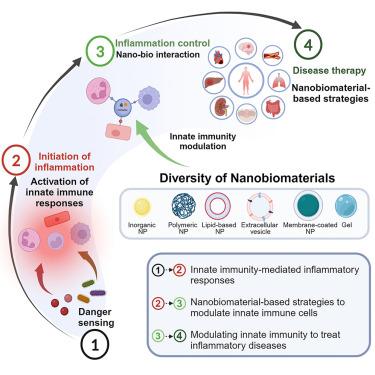
|
The acute inflammatory response is an inherent protective mechanism, and its unsuccessful resolution can contribute to disease pathogenesis and potentially lead to death. Innate immune cells are the first line of host defenders and play a substantial role in inflammation initiation, amplification, resolution, or subsequent disease progression. As the resolution of inflammation is an active and highly regulated process, modulating innate immune cells, including neutrophils, monocytes and macrophages, and endothelial cells, and their interactions offer opportunities to control excessive inflammation. Nanobiomaterials have shown superior therapeutic potential in inflammation-related diseases by manipulating inflammatory responses because nanobiomaterials can target and interact with innate immune cells. Versatile nanobiomaterials can be designed for targeted modulation of specific innate immune responses. Nanopro-resolving medicines have been prepared both with pro-resolving lipid mediators and peptides, each demonstrating active resolution of inflammation in animal disease models. Here, we review innovative nanobiomaterials for modulating innate immunity and alleviating inflammation. We summarize strategies combining the design of nanobiomaterials with the nano-bio interaction for modulating innate immune profiles and propelling the advancement of nanobiomaterials for inflammatory disease treatments. We also propose the future perspectives and translational challenges of nanobiomaterials that need to be overcome in this swiftly rising field.
中文翻译:

用于控制炎症消退的先天免疫调节纳米生物材料
急性炎症反应是一种固有的保护机制,其不成功的消退可导致疾病发病机制并可能导致死亡。先天免疫细胞是宿主防御者的第一线,在炎症的发生、扩增、消退或随后的疾病进展中发挥着重要作用。由于炎症的消退是一个积极且高度调节的过程,因此调节先天免疫细胞(包括中性粒细胞、单核细胞和巨噬细胞)以及内皮细胞及其相互作用为控制过度炎症提供了机会。纳米生物材料通过操纵炎症反应在炎症相关疾病中显示出卓越的治疗潜力,因为纳米生物材料可以靶向先天免疫细胞并与之相互作用。多功能纳米生物材料可用于靶向调节特异性先天免疫反应。已经制备了含有促分解脂质介质和肽的 Nanopro-resolution 药物,每种药物在动物疾病模型中都显示出对炎症的积极消退。在这里,我们回顾了用于调节先天免疫和缓解炎症的创新纳米生物材料。我们总结了将纳米生物材料的设计与纳米生物相互作用相结合的策略,以调节先天免疫特征并推动纳米生物材料在炎症性疾病治疗中的进步。我们还提出了在这个迅速崛起的领域需要克服的纳米生物材料的未来前景和转化挑战。
更新日期:2024-11-06
中文翻译:

用于控制炎症消退的先天免疫调节纳米生物材料
急性炎症反应是一种固有的保护机制,其不成功的消退可导致疾病发病机制并可能导致死亡。先天免疫细胞是宿主防御者的第一线,在炎症的发生、扩增、消退或随后的疾病进展中发挥着重要作用。由于炎症的消退是一个积极且高度调节的过程,因此调节先天免疫细胞(包括中性粒细胞、单核细胞和巨噬细胞)以及内皮细胞及其相互作用为控制过度炎症提供了机会。纳米生物材料通过操纵炎症反应在炎症相关疾病中显示出卓越的治疗潜力,因为纳米生物材料可以靶向先天免疫细胞并与之相互作用。多功能纳米生物材料可用于靶向调节特异性先天免疫反应。已经制备了含有促分解脂质介质和肽的 Nanopro-resolution 药物,每种药物在动物疾病模型中都显示出对炎症的积极消退。在这里,我们回顾了用于调节先天免疫和缓解炎症的创新纳米生物材料。我们总结了将纳米生物材料的设计与纳米生物相互作用相结合的策略,以调节先天免疫特征并推动纳米生物材料在炎症性疾病治疗中的进步。我们还提出了在这个迅速崛起的领域需要克服的纳米生物材料的未来前景和转化挑战。






























 京公网安备 11010802027423号
京公网安备 11010802027423号