Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optical tweezer-assisted cell pairing and fusion for somatic cell nuclear transfer within an open microchannel
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-11-06 , DOI: 10.1039/d4lc00561a
Yidi Zhang 1, 2 , Han Zhao 2 , Zhenlin Chen 2 , Zhen Liu 2 , Hanjin Huang 2 , Yun Qu 2 , Yaowei Liu 1, 3 , Mingzhu Sun 1, 3 , Dong Sun 2 , Xin Zhao 1, 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-11-06 , DOI: 10.1039/d4lc00561a
Yidi Zhang 1, 2 , Han Zhao 2 , Zhenlin Chen 2 , Zhen Liu 2 , Hanjin Huang 2 , Yun Qu 2 , Yaowei Liu 1, 3 , Mingzhu Sun 1, 3 , Dong Sun 2 , Xin Zhao 1, 3
Affiliation
|
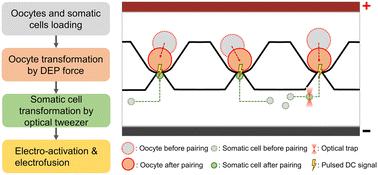
Somatic cell nuclear transfer (SCNT), referred to as somatic cell cloning, is a pivotal biotechnological technique utilized across various applications. Although robotic SCNT is currently available, the subsequent oocyte electrical activation/reconstructed embryo electrofusion is still manually completed by skilled operators, presenting challenges in efficient manipulation due to the uncontrollable positioning of the reconstructed embryo. This study introduces a robotic SCNT-electrofusion system to enable high-precision batch SCNT cloning. The proposed system integrates optical tweezers and microfluidic technologies. An optical tweezer is employed to facilitate somatic cells in precisely reaching the fusion site, and a specific polydimethylsiloxane (PDMS) chip is designed to assist in positioning and pairing oocytes and somatic cells. Enhancement in the electric field distribution between two parallel electrodes by PDMS pillars significantly reduces the required external voltage for electrofusion/electrical activation. We employed porcine oocytes and porcine fetal fibroblasts for SCNT experiments. The experimental results show that 90.56% of oocytes successfully paired with somatic cells to form reconstructed embryos, 76.43% of the reconstructed embryos successfully fused, and 70.55% of these embryos underwent cleavage. It demonstrates that the present system achieves the robotic implementation of oocyte electrical activation/reconstructed embryo electrofusion. By leveraging the advantages of batch operations using microfluidics, it proposes an innovative robotic cloning procedure that scales embryo cloning.
中文翻译:

光学镊子辅助细胞配对和融合,用于开放微通道内的体细胞核转移
体细胞核移植 (SCNT),简称体细胞克隆,是一种用于各种应用的关键生物技术技术。尽管目前可以使用机器人 SCNT,但随后的卵母细胞电激活/重建胚胎电融合仍由熟练的操作员手动完成,由于重建胚胎的定位无法控制,因此对有效操作提出了挑战。本研究介绍了一种机器人 SCNT 电融合系统,以实现高精度批量 SCNT 克隆。拟议的系统集成了光镊和微流体技术。采用光镊促进体细胞精确到达融合位点,并设计了特定的聚二甲基硅氧烷 (PDMS) 芯片来协助定位和配对卵母细胞和体细胞。PDMS 柱增强两个平行电极之间的电场分布,显著降低了电熔/电激活所需的外部电压。我们使用猪卵母细胞和猪胎儿成纤维细胞进行 SCNT 实验。实验结果表明,90.56% 的卵母细胞成功与体细胞配对形成重建胚胎,76.43% 的重建胚胎成功融合,70.55% 的胚胎发生卵裂。它表明,该系统实现了卵母细胞电激活/重建胚胎电融合的机器人实施。通过利用微流体的批量操作的优势,它提出了一种创新的机器人克隆程序,可以扩大胚胎克隆的规模。
更新日期:2024-11-06
中文翻译:

光学镊子辅助细胞配对和融合,用于开放微通道内的体细胞核转移
体细胞核移植 (SCNT),简称体细胞克隆,是一种用于各种应用的关键生物技术技术。尽管目前可以使用机器人 SCNT,但随后的卵母细胞电激活/重建胚胎电融合仍由熟练的操作员手动完成,由于重建胚胎的定位无法控制,因此对有效操作提出了挑战。本研究介绍了一种机器人 SCNT 电融合系统,以实现高精度批量 SCNT 克隆。拟议的系统集成了光镊和微流体技术。采用光镊促进体细胞精确到达融合位点,并设计了特定的聚二甲基硅氧烷 (PDMS) 芯片来协助定位和配对卵母细胞和体细胞。PDMS 柱增强两个平行电极之间的电场分布,显著降低了电熔/电激活所需的外部电压。我们使用猪卵母细胞和猪胎儿成纤维细胞进行 SCNT 实验。实验结果表明,90.56% 的卵母细胞成功与体细胞配对形成重建胚胎,76.43% 的重建胚胎成功融合,70.55% 的胚胎发生卵裂。它表明,该系统实现了卵母细胞电激活/重建胚胎电融合的机器人实施。通过利用微流体的批量操作的优势,它提出了一种创新的机器人克隆程序,可以扩大胚胎克隆的规模。







































 京公网安备 11010802027423号
京公网安备 11010802027423号