当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Dehydrogenation of Ethane on Dynamically Rearranging Supported Chloride Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-28 , DOI: 10.1021/ja505411s
Christian A. Gärtner 1 , André C. van Veen 1, 2 , Johannes A. Lercher 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-28 , DOI: 10.1021/ja505411s
Christian A. Gärtner 1 , André C. van Veen 1, 2 , Johannes A. Lercher 1
Affiliation
|
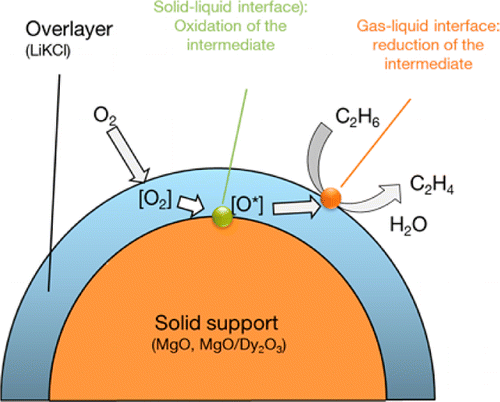
Ethane is oxidatively dehydrogenated with a selectivity up to 95% on catalysts comprising a mixed molten alkali chloride supported on a mildly redox-active Dy2O3-doped MgO. The reactive oxyanionic OCl(-) species acting as active sites are catalytically formed by oxidation of Cl(-) at the MgO surface. Under reaction conditions this site is regenerated by O2, dissolving first in the alkali chloride melt, and in the second step dissociating and replenishing the oxygen vacancies on MgO. The oxyanion reactively dehydrogenates ethane at the melt-gas phase interface with nearly ideal selectivity. Thus, the reaction is concluded to proceed via two coupled steps following a Mars-van-Krevelen-mechanism at the solid-liquid and gas-liquid interface. The dissociation of O2 and/or the oxidation of Cl(-) at the melt-solid interface is concluded to have the lowest forward rate constants. The compositions of the oxide core and the molten chloride shell control the catalytic activity via the redox potential of the metal oxide and of the OCl(-). Traces of water may be present in the molten chloride under reaction conditions, but the specific impact of this water is not obvious at present. The spatial separation of oxygen and ethane activation sites and the dynamic rearrangement of the surface anions and cations, preventing the exposure of coordinatively unsaturated cations, are concluded to be the origin of the surprisingly high olefin selectivity.
中文翻译:

动态重排负载氯化物催化剂上乙烷的氧化脱氢
乙烷在催化剂上以高达 95% 的选择性进行氧化脱氢,该催化剂包含负载在轻度氧化还原活性 Dy2O3 掺杂的 MgO 上的混合熔融碱金属氯化物。充当活性位点的活性氧阴离子 OCl(-) 物种是通过在 MgO 表面氧化 Cl(-) 催化形成的。在反应条件下,该位点由 O2 再生,首先溶解在碱金属氯化物熔体中,然后在第二步中解离和补充 MgO 上的氧空位。氧阴离子在熔体-气相界面以近乎理想的选择性对乙烷进行反应性脱氢。因此,该反应在固-液和气-液界面按照 Mars-van-Krevelen 机制通过两个耦合步骤进行。在熔体-固体界面处 O2 的解离和/或 Cl(-) 的氧化被认为具有最低的正向速率常数。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。
更新日期:2014-08-28
中文翻译:

动态重排负载氯化物催化剂上乙烷的氧化脱氢
乙烷在催化剂上以高达 95% 的选择性进行氧化脱氢,该催化剂包含负载在轻度氧化还原活性 Dy2O3 掺杂的 MgO 上的混合熔融碱金属氯化物。充当活性位点的活性氧阴离子 OCl(-) 物种是通过在 MgO 表面氧化 Cl(-) 催化形成的。在反应条件下,该位点由 O2 再生,首先溶解在碱金属氯化物熔体中,然后在第二步中解离和补充 MgO 上的氧空位。氧阴离子在熔体-气相界面以近乎理想的选择性对乙烷进行反应性脱氢。因此,该反应在固-液和气-液界面按照 Mars-van-Krevelen 机制通过两个耦合步骤进行。在熔体-固体界面处 O2 的解离和/或 Cl(-) 的氧化被认为具有最低的正向速率常数。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。氧化物核和熔融氯化物壳的组成通过金属氧化物和 OCl(-) 的氧化还原电位控制催化活性。在反应条件下,熔融氯化物中可能存在微量水,但该水的具体影响目前尚不明显。氧和乙烷活化位点的空间分离以及表面阴离子和阳离子的动态重排,防止了配位不饱和阳离子的暴露,被认为是令人惊讶的高烯烃选择性的起源。

































 京公网安备 11010802027423号
京公网安备 11010802027423号