当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conduction channel creation by interstitial site engineering in otherwise insulating Na6ZnS4 for Na-conducting solid-state electrolytes
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-05 , DOI: 10.1016/j.ensm.2024.103889 Seol Yeon Kang, Woon-Bae Park, Jung Yong Seo, Kee-Sun Sohn, Young-Kook Lee, Joon Seop Kwak, Myoungho Pyo
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-05 , DOI: 10.1016/j.ensm.2024.103889 Seol Yeon Kang, Woon-Bae Park, Jung Yong Seo, Kee-Sun Sohn, Young-Kook Lee, Joon Seop Kwak, Myoungho Pyo
|
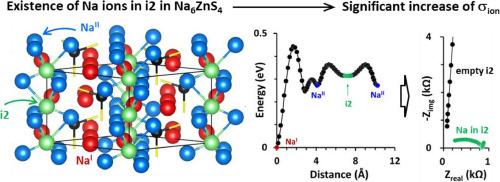
Na5.6 Zn0.6 Ga0.4 S4 , which retains the crystalline structure of its parent form Na6 ZnS4 , is described as a new class of Na-conducting solid-state electrolytes (SSEs) for all-solid-state batteries. We demonstrate that while Na6 ZnS4 is ionically insulating (1.4 nS cm-1 ), Ga-substitution results in an astonishing improvement of ionic conductivity (σion ) to 70.1 μS cm-1 , making Na5.6 Zn0.6 Ga0.4 S4 a practical SSE. This dramatic increase in σion (5 × 104 fold) is associated with an increased Na+ occupancy in interstitial sites as ‘x’ increases in Na6-x Zn1-x Gax S4 , where interstitial Na ions facilitate long-range Na+ conduction, which is otherwise immobile. Ga-substitution also results in phase-pure Na6-x Zn1-x Gax S4 , contributing at least partially to the enhancement of σion . Furthermore, Na6-x Zn1-x Gax S4 exhibits neither releasing H2 S gas nor compromising its crystalline structure for several hours under ambient conditions. Ga-substitution also enhances electrochemical stability. While the anodic limit remains largely unchanged, the cathodic limit is significantly lowered from 0.99 V vs. Na2 Sn in Na6 ZnS4 to 0.35 V in Na5.6 Zn0.6 Ga0.4 S4 , resulting in stable Na alloying/dealloying reactions in a symmetric Na2 Sn ‖ Na2 Sn cell. These findings are comprehensively supported by various experimental and theoretical methods. Finally, we construct a full cell (Na2 Sn ‖ TiS2 ) and demonstrate the practicality of Na5.6 Zn0.6 Ga0.4 S4 as a promising SSE in all solid-state Na ion batteries.
中文翻译:

通过间隙位点工程在用于 Na 导电固态电解质的绝缘 Na6ZnS4 中创建导电通道
Na5.6Zn0.6Ga0.4S4 保留了其母体 Na6ZnS4 的晶体结构,被描述为一类用于全固态电池的新型 Na 导电固态电解质 (SSE)。我们证明,虽然 Na6ZnS4 具有离子绝缘性 (1.4 nS cm-1),但 Ga-取代导致离子电导率 (σion) 惊人地提高到 70.1 μS cm-1,使 Na5.6Zn0.6Ga0.4S4 成为实用的 SSE。σion 的这种急剧增加(5 × 104 倍)与间质部位的 Na+ 占有率增加有关,因为 Na6-xZn1-xGaxS4 的“x”增加,其中间质 Na 离子促进长程 Na+ 传导,否则它是不动的。Ga-取代还导致相纯 Na6-xZn1-xGaxS4,至少部分有助于 σion 的增强。此外,Na6-xZn1-xGaxS4 在环境条件下数小时内既不释放 H2S 气体,也不损害其晶体结构。Ga-取代还增强了电化学稳定性。虽然阳极极限基本保持不变,但阴极极限从 Na6ZnS4 中的 0.99 V vs. Na2Sn 显著降低到 Na5.6Zn0.6Ga0.4S4 中的 0.35 V,从而在对称的 Na2Sn ‖ Na2Sn 电池中产生稳定的 Na 合金化/脱合金反应。这些发现得到了各种实验和理论方法的全面支持。最后,我们构建了一个全电池 (Na2Sn ‖ TiS2) 并证明了 Na5.6Zn0.6Ga0.4S4 在所有固态 Na 离子电池中作为有前途的 SSE 的实用性。
更新日期:2024-11-05
中文翻译:

通过间隙位点工程在用于 Na 导电固态电解质的绝缘 Na6ZnS4 中创建导电通道
Na5.6Zn0.6Ga0.4S4 保留了其母体 Na6ZnS4 的晶体结构,被描述为一类用于全固态电池的新型 Na 导电固态电解质 (SSE)。我们证明,虽然 Na6ZnS4 具有离子绝缘性 (1.4 nS cm-1),但 Ga-取代导致离子电导率 (σion) 惊人地提高到 70.1 μS cm-1,使 Na5.6Zn0.6Ga0.4S4 成为实用的 SSE。σion 的这种急剧增加(5 × 104 倍)与间质部位的 Na+ 占有率增加有关,因为 Na6-xZn1-xGaxS4 的“x”增加,其中间质 Na 离子促进长程 Na+ 传导,否则它是不动的。Ga-取代还导致相纯 Na6-xZn1-xGaxS4,至少部分有助于 σion 的增强。此外,Na6-xZn1-xGaxS4 在环境条件下数小时内既不释放 H2S 气体,也不损害其晶体结构。Ga-取代还增强了电化学稳定性。虽然阳极极限基本保持不变,但阴极极限从 Na6ZnS4 中的 0.99 V vs. Na2Sn 显著降低到 Na5.6Zn0.6Ga0.4S4 中的 0.35 V,从而在对称的 Na2Sn ‖ Na2Sn 电池中产生稳定的 Na 合金化/脱合金反应。这些发现得到了各种实验和理论方法的全面支持。最后,我们构建了一个全电池 (Na2Sn ‖ TiS2) 并证明了 Na5.6Zn0.6Ga0.4S4 在所有固态 Na 离子电池中作为有前途的 SSE 的实用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号