当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
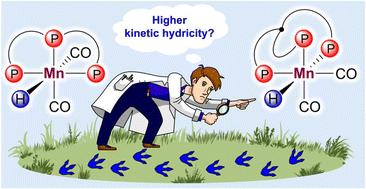
Influence of triphosphine ligand coordination geometry in Mn(I) hydride complexes [(P∩P∩P)(CO)2MnH] on their kinetic hydricity
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-06 , DOI: 10.1039/d4dt02496f Sergey A. Kovalenko, Ekaterina S. Gulyaeva, Elena S. Osipova, Oleg A. Filippov, Anastasia A. Danshina, Laure Vendier, Nikolay V. Kireev, Ivan A. Godovikov, Yves Canac, Dmitry A. Valyaev, Natalia V. Belkova, Elena S. Shubina
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-06 , DOI: 10.1039/d4dt02496f Sergey A. Kovalenko, Ekaterina S. Gulyaeva, Elena S. Osipova, Oleg A. Filippov, Anastasia A. Danshina, Laure Vendier, Nikolay V. Kireev, Ivan A. Godovikov, Yves Canac, Dmitry A. Valyaev, Natalia V. Belkova, Elena S. Shubina
|
Octahedral Mn(I) complexes bearing tridentate donor ligands [(L∩L′∩L′′)(CO)2MnX] have recently emerged as major players in catalytic (de)hydrogenation processes. While most of these systems are still based on structurally rigid pincer scaffolds imposing a meridional coordination mode, for some more flexible tridentate ligands a facial arrangement of donor moieties becomes possible. Accordingly, the geometry of the corresponding Mn(I) hydrides [(L∩L′∩L′′)(CO)2MnH] directly involved in the catalytic processes, namely the nature of the donor extremity located in the trans-position of the hydride (CO and L for mer- and fac-configurations, respectively) may influence their hydride transfer ability. Herein, low-temperature IR and NMR spectroscopy studies of two model Mn(I) complexes, mer-[(L1)(CO)2MnH] and fac-[(L2)(CO)2MnH], bearing similar triphosphine ligands (L1 = PhP(CH2CH2PPh2)2; L2 = MeC(CH2PPh2)3) in the presence of B(C6F5)3 as the H− abstractor revealed for the first time a higher kinetic hydricity of the tripodal system. Even for the pincer complex, hydride transfer proceeds from the non-covalent adduct fac-[(L1)(CO)2MnH]⋯B(C6F5)3 with the facial geometry arising from the mer-to-fac isomerization of the initial mer-[(L1)(CO)2MnH]. The higher reactivity of the fac-hydride derivatives was found to be consistent with the catalytic performance of the corresponding Mn(I) bromide complexes in the benchmark ester hydrosilylation.
中文翻译:

Mn(I) 氢化物配合物中三磷氢配位几何结构 [(P∩P∩P)(CO)2MnH] 对其动力学水合度的影响
带有三齿供体配体 [(L∩L′∩L′′)(CO)2MnX] 的八面体 Mn() 配合物最近已成为催化(脱)氢化过程的主要参与者。虽然这些系统中的大多数仍然基于结构刚性的钳式支架,施加经向协调模式,但对于一些更灵活的三齿配体,供体部分的面部排列成为可能。因此,直接参与催化过程的相应 Mn() 氢化物 [(L∩L′∩L′′)(CO)2MnH] 的几何形状,即位于氢化物转位中的供体末端的性质(分别为 CO 和 L 用于 mer 和 fac 构型)可能会影响它们的氢化物转移能力。本文对两种模型 Mn() 配合物 mer-[(L1)(CO)2MnH] 和 fac-[(L2)(CO)2MnH],具有相似的三磷氢配体(L1 = PhP(CH2CH2PPh2)2;L2 = MeC(CH2PPh2)3) 在 B(C6F5)3 作为 H− 抽象器的存在下,首次揭示了三脚体系统的更高动力学水力。 即使对于钳形配合物,氢化物转移也来自非共价加合物 fac-[(L1)(CO)2MnH]⋯B(C6F5)3,其面部几何形状是由初始 mer-[(L1)(CO)2MnH] 的 mer-fac 异构化产生的。发现 fac-hydride 衍生物的较高反应性与基准酯氢化硅烷化中相应 Mn() 溴化物络合物的催化性能一致。
更新日期:2024-11-06
中文翻译:

Mn(I) 氢化物配合物中三磷氢配位几何结构 [(P∩P∩P)(CO)2MnH] 对其动力学水合度的影响
带有三齿供体配体 [(L∩L′∩L′′)(CO)2MnX] 的八面体 Mn() 配合物最近已成为催化(脱)氢化过程的主要参与者。虽然这些系统中的大多数仍然基于结构刚性的钳式支架,施加经向协调模式,但对于一些更灵活的三齿配体,供体部分的面部排列成为可能。因此,直接参与催化过程的相应 Mn() 氢化物 [(L∩L′∩L′′)(CO)2MnH] 的几何形状,即位于氢化物转位中的供体末端的性质(分别为 CO 和 L 用于 mer 和 fac 构型)可能会影响它们的氢化物转移能力。本文对两种模型 Mn() 配合物 mer-[(L1)(CO)2MnH] 和 fac-[(L2)(CO)2MnH],具有相似的三磷氢配体(L1 = PhP(CH2CH2PPh2)2;L2 = MeC(CH2PPh2)3) 在 B(C6F5)3 作为 H− 抽象器的存在下,首次揭示了三脚体系统的更高动力学水力。 即使对于钳形配合物,氢化物转移也来自非共价加合物 fac-[(L1)(CO)2MnH]⋯B(C6F5)3,其面部几何形状是由初始 mer-[(L1)(CO)2MnH] 的 mer-fac 异构化产生的。发现 fac-hydride 衍生物的较高反应性与基准酯氢化硅烷化中相应 Mn() 溴化物络合物的催化性能一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号