当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Early Process Development of an LPAR1 Antagonist, GS-2278
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.oprd.4c00369 Nathaniel Kadunce, Anna M. Wagner, Jeromy Cottell, Kathy Dao, Darryl D. Dixon, Blanka M. Hodur, Dane Holte, Michael A. Ischay, Jihun Kang, Seongtaek Kim, Young Ho Kim, Seung Moh Koo, Willard Lew, Lucas Man, Kashi Reddy Methuku, Henry Morrison, Patrick D. Parker, David A. Siler, Chloe Y. Wong
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.oprd.4c00369 Nathaniel Kadunce, Anna M. Wagner, Jeromy Cottell, Kathy Dao, Darryl D. Dixon, Blanka M. Hodur, Dane Holte, Michael A. Ischay, Jihun Kang, Seongtaek Kim, Young Ho Kim, Seung Moh Koo, Willard Lew, Lucas Man, Kashi Reddy Methuku, Henry Morrison, Patrick D. Parker, David A. Siler, Chloe Y. Wong
|
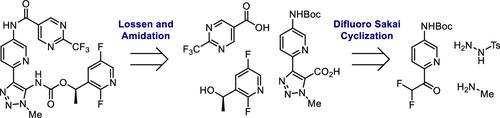
(R)-1-(2,5-Difluoropyridin-3-yl)ethyl(1-methyl-4-(5-(2-(trifluoromethyl)pyrimidine-5-carboxamido)pyridin-2-yl)-1H-1,2,3-triazol-5-yl)carbamate (GS-2278) is a lysophosphatidic acid receptor 1 antagonist under development for the treatment of idiopathic pulmonary fibrosis. GS-2278 is assembled in a 9-step sequence. Initially, 2-bromo-5-fluoropyridine is metalated and trapped with ethyl difluoroacetate. Then, after condensation with tosyl hydrazide, Sakai cyclization with methylamine, and carboxylation with carbon dioxide, the triazole carboxylic acid core is generated. For the final assembly, the core is elaborated through a two-step hydroxamic acid formation and Lossen rearrangement to form an isocyanate which is trapped in situ by a chiral alcohol. The resulting carbamate is Boc-deprotected and subjected to amide coupling with a pyrimidine carboxylic acid to yield the active pharmaceutical ingredient. Process development was conducted to determine reaction and isolation conditions to enable scale-ups to support preclinical and early clinical studies. This paper focuses on the development of conditions from the medicinal chemistry route to the Ph 1 manufacturing route.
中文翻译:

LPAR1 拮抗剂 GS-2278 的早期工艺开发
(R)-1-(2,5-二氟吡啶-3-基)乙基(1-甲基-4-(5-(2-(三氟甲基)嘧啶-5-甲酰胺基)吡啶-2-基)-1 H-1,2,3-三唑-5-基)氨基甲酸酯(GS-2278)是一种溶血磷脂酸受体1拮抗剂,正在开发中,用于治疗特发性肺纤维化。GS-2278 以 9 步顺序组装。最初,2-溴-5-氟吡啶被金属化并被二氟乙酸乙酯捕获。然后,与甲苯肼缩合,用甲胺进行 Sakai 环化,与二氧化碳进行羧化反应后,生成三唑羧酸核心。对于最终组装,通过两步异羟肟酸形成和 Lossen 重排对核心进行精制,形成异氰酸酯,该异氰酸酯被手性醇原位捕获。所得氨基甲酸酯经过 Boc 脱保护,并与嘧啶羧酸进行酰胺偶联,得到活性药物成分。进行工艺开发以确定反应和分离条件,以便进行放大以支持临床前和早期临床研究。本文重点介绍了从药物化学路线到 Ph 1 制造路线的条件发展。
更新日期:2024-11-05
中文翻译:

LPAR1 拮抗剂 GS-2278 的早期工艺开发
(R)-1-(2,5-二氟吡啶-3-基)乙基(1-甲基-4-(5-(2-(三氟甲基)嘧啶-5-甲酰胺基)吡啶-2-基)-1 H-1,2,3-三唑-5-基)氨基甲酸酯(GS-2278)是一种溶血磷脂酸受体1拮抗剂,正在开发中,用于治疗特发性肺纤维化。GS-2278 以 9 步顺序组装。最初,2-溴-5-氟吡啶被金属化并被二氟乙酸乙酯捕获。然后,与甲苯肼缩合,用甲胺进行 Sakai 环化,与二氧化碳进行羧化反应后,生成三唑羧酸核心。对于最终组装,通过两步异羟肟酸形成和 Lossen 重排对核心进行精制,形成异氰酸酯,该异氰酸酯被手性醇原位捕获。所得氨基甲酸酯经过 Boc 脱保护,并与嘧啶羧酸进行酰胺偶联,得到活性药物成分。进行工艺开发以确定反应和分离条件,以便进行放大以支持临床前和早期临床研究。本文重点介绍了从药物化学路线到 Ph 1 制造路线的条件发展。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号