当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting Catalytic Steric Hindrance for Enhanced Tishchenko Polymerization: Toward Biorenewable Aromatic Polyesters
Macromolecules ( IF 5.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.macromol.4c01955 Hongru Qiang, Xue Liang, Wenli Wang, Jiayun Jiang, Jianrui Li, Kai Hong, Jianzhong Du, Yunqing Zhu
Macromolecules ( IF 5.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.macromol.4c01955 Hongru Qiang, Xue Liang, Wenli Wang, Jiayun Jiang, Jianrui Li, Kai Hong, Jianzhong Du, Yunqing Zhu
|
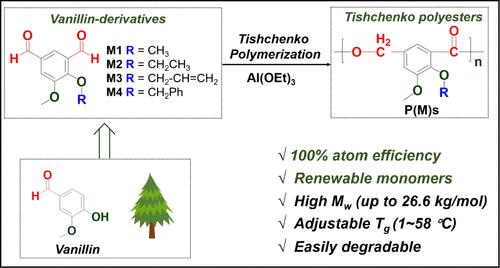
Tishchenko reaction represents a highly atom-efficient method for synthesizing esters via aldehydes disproportionation, which holds significant promise in sustainable chemistry for polymerizing biorenewable dialdehydes into polyesters. However, previous Tishchenko polymerization attempts merely yielded oligomers with very low molecular weights due to intramolecular cyclization. To address this important challenge, we propose an efficient approach to leverage catalytic steric hindrance to achieve higher molecular weights. The investigation of six catalysts revealed that Al(OEt)3, with its three ethoxy groups initiating, imparts significant steric hindrance around the metal center and effectively prevents cyclization termination at the initial stage of polymerization, resulting in aromatic polyesters with a record Mw of 26.6 kg/mol. This breakthrough signals the potential of enhancing catalytic steric hindrance to overcome the low-molecular-weight limitation of Tishchenko polymerization. Building upon this discovery, we synthesized a series of biorenewable meta-substituted dialdehyde monomers (M1–M4) derived from vanillin to afford aromatic polyesters P(M1)–P(M4). These polymers offer tunable thermal properties through molecular weight and side chain flexibility adjustments, exhibiting high thermal stability (Td,5% > 208 °C) and controllable glass transition temperatures (1–58 °C), and are degradable under mild conditions. This work highlights the importance of developing new methods to utilize readily available bioderived molecules, which is of great value for the sustainable economy.
中文翻译:

利用催化空间位阻增强 Tishchenko 聚合:迈向生物可再生芳香族聚酯
Tishchenko 反应代表了一种通过醛歧化合成酯的高原子效率方法,这在将生物可再生二醛聚合成聚酯的可持续化学中具有重要前景。然而,由于分子内环化,以前的 Tishchenko 聚合尝试仅产生分子量非常低的低聚物。为了应对这一重要挑战,我们提出了一种利用催化空间位阻来实现更高分子量的有效方法。对六种催化剂的调查表明,Al(OEt)3 及其三个乙氧基引发,在金属中心周围施加显着的空间位阻,并有效防止在聚合初始阶段环化终止,从而产生创纪录的 Mw 为 26.6 kg/mol 的芳香族聚酯。这一突破标志着增强催化空间位阻的潜力,以克服 Tishchenko 聚合的低分子量限制。基于这一发现,我们合成了一系列源自香兰素的生物可替代间取代二醛单体 (M1-M4),以获得芳香族聚酯 P(M1)-P(M4)。这些聚合物通过分子量和侧链柔韧性调整提供可调的热性能,表现出高热稳定性 (Td,5% > 208 °C) 和可控的玻璃化转变温度 (1–58 °C),并且在温和条件下可降解。这项工作强调了开发新方法以利用现成的生物衍生分子的重要性,这对可持续经济具有重要价值。
更新日期:2024-11-05
中文翻译:

利用催化空间位阻增强 Tishchenko 聚合:迈向生物可再生芳香族聚酯
Tishchenko 反应代表了一种通过醛歧化合成酯的高原子效率方法,这在将生物可再生二醛聚合成聚酯的可持续化学中具有重要前景。然而,由于分子内环化,以前的 Tishchenko 聚合尝试仅产生分子量非常低的低聚物。为了应对这一重要挑战,我们提出了一种利用催化空间位阻来实现更高分子量的有效方法。对六种催化剂的调查表明,Al(OEt)3 及其三个乙氧基引发,在金属中心周围施加显着的空间位阻,并有效防止在聚合初始阶段环化终止,从而产生创纪录的 Mw 为 26.6 kg/mol 的芳香族聚酯。这一突破标志着增强催化空间位阻的潜力,以克服 Tishchenko 聚合的低分子量限制。基于这一发现,我们合成了一系列源自香兰素的生物可替代间取代二醛单体 (M1-M4),以获得芳香族聚酯 P(M1)-P(M4)。这些聚合物通过分子量和侧链柔韧性调整提供可调的热性能,表现出高热稳定性 (Td,5% > 208 °C) 和可控的玻璃化转变温度 (1–58 °C),并且在温和条件下可降解。这项工作强调了开发新方法以利用现成的生物衍生分子的重要性,这对可持续经济具有重要价值。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号