GeroScience ( IF 5.3 ) Pub Date : 2024-11-05 , DOI: 10.1007/s11357-024-01406-7 Carlo Gaetano, Sandra Atlante, Michela Gottardi Zamperla, Veronica Barbi, Davide Gentilini, Barbara Illi, Marco Malavolta, Fabio Martelli, Antonella Farsetti
|
The COVID-19 pandemic has left a lasting legacy on human health, extending beyond the acute phase of infection. This article explores the evidence suggesting that SARS-CoV-2 infection can induce persistent epigenetic modifications, particularly in DNA methylation patterns, with potential long-term consequences for individuals’ health and aging trajectories. The review discusses the potential of DNA methylation-based biomarkers, such as epigenetic clocks, to identify individuals at risk for accelerated aging and tailor personalized interventions. Integrating epigenetic clock analysis into clinical management could mark a new era of personalized treatment for COVID-19, possibly helping clinicians to understand patient susceptibility to severe outcomes and establish preventive strategies. Several valuable reviews address the role of epigenetics in infectious diseases, including the Sars-CoV-2 infection. However, this article provides an original overview of the current understanding of the epigenetic dimensions of COVID-19, offering insights into the long-term health implications of the pandemic. While acknowledging the limitations of current data, we emphasize the need for future research to unravel the precise mechanisms underlying COVID-19-induced epigenetic changes and to explore potential approaches to target these modifications.
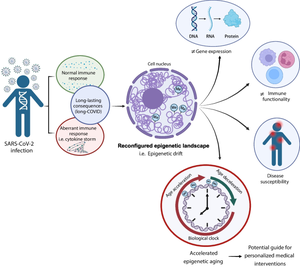
Graphical Abstract: Impact of SARS-CoV-2 infection on the epigenetic landscape and individual response
Following SARS-CoV-2 infection, individuals may develop either a normal immune response or an aberrant one, such as a cytokine storm. Both scenarios can result in long-lasting consequences, known as “long COVID.” This condition can reshape the epigenetic landscape by altering DNA methylation patterns, contributing to the “epigenetic drift.” This drift, further influenced by various factors, can lead to changes in gene expression, immune functionality, and disease susceptibility. One significant consequence of the epigenetic drift is the acceleration of biological aging, which can profoundly impact personalized medical interventions. Created with BioRender.com.
中文翻译:

COVID-19 遗留问题:对人类 DNA 甲基化组和治疗前景的影响
COVID-19 大流行对人类健康留下了持久的影响,其影响超出了感染的急性期。本文探讨了表明 SARS-CoV-2 感染可以诱导持续性表观遗传修饰的证据,尤其是在 DNA 甲基化模式中,对个体的健康和衰老轨迹具有潜在的长期影响。本综述讨论了基于 DNA 甲基化的生物标志物(例如表观遗传时钟)的潜力,以识别有加速衰老风险的个体并定制个性化干预措施。将表观遗传时钟分析整合到临床管理中可能标志着 COVID-19 个性化治疗的新时代,可能有助于临床医生了解患者对严重结果的易感性并制定预防策略。几篇有价值的综述讨论了表观遗传学在传染病(包括 Sars-CoV-2 感染)中的作用。然而,本文对当前对 COVID-19 表观遗传维度的理解进行了原始概述,为大流行对健康的长期影响提供了见解。在承认当前数据的局限性的同时,我们强调未来研究需要揭示 COVID-19 诱导的表观遗传变化的确切机制,并探索针对这些修饰的潜在方法。
图形摘要:SARS-CoV-2 感染对表观遗传学景观和个体反应的影响
感染 SARS-CoV-2 后,个体可能会出现正常的免疫反应或异常的免疫反应,例如细胞因子风暴。这两种情况都可能导致长期后果,称为“长期 COVID”。这种情况可以通过改变 DNA 甲基化模式来重塑表观遗传景观,从而导致“表观遗传漂移”。这种漂移,进一步受到各种因素的影响,可导致基因表达、免疫功能和疾病易感性发生变化。表观遗传漂移的一个重要后果是生物衰老的加速,这会对个性化医疗干预产生深远影响。使用 BioRender.com 创建。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号