当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Target Ligand Separation and Identification of Isoforsythiaside as a Histone Lysine-Specific Demethylase 1 Covalent Inhibitor Against Breast Cancer Metastasis
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.jmedchem.4c02277 Mengzhen Gu, Xiaoqing Xu, Xiaoping Wang, Yun Wang, Yu Zhao, Xiaoxian Hu, Lu Zhu, Zhenzhong Deng, Chao Han
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.jmedchem.4c02277 Mengzhen Gu, Xiaoqing Xu, Xiaoping Wang, Yun Wang, Yu Zhao, Xiaoxian Hu, Lu Zhu, Zhenzhong Deng, Chao Han
|
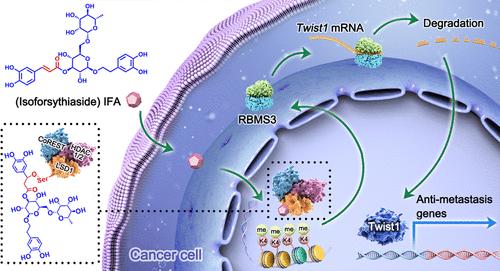
Histone lysine-specific demethylase 1 (LSD1) is hyperactive in breast cancer, which is associated with the metastasis of the tumor. Current irreversible LSD1 inhibitors are all synthesized by covalently binding to the flavin adenine dinucleotide cofactor, which often have side effects due to the high affinity for a variety of targets. Here, we identified isoforsythiaside (IFA), a natural phenylpropanoid glycoside isolated from Forsythia suspensa, as a novel covalent inhibitor of LSD1. The target ligand fishing technique and LC–MS/MS analysis identified that IFA could covalently bind to the Ser817 residue of LSD1 by α,β-unsaturated ketone moiety to block the amine oxidase-like domain of LSD1. Moreover, RBMS3/Twist1/MMP2, the downstream signaling pathway of LSD1, was activated after IFA treatment to inhibit the metastasis of MDA-MB-231 cells in vitro and in vivo. This study provided novel molecular templates for development of LSD1 covalence-binding inhibitor and laid a foundation for developing agents against breast carcinoma metastasis for targeting LSD1.
中文翻译:

异连翘苷作为组蛋白赖氨酸特异性去甲基化酶 1 共价抑制剂的靶向配体分离和鉴定,对抗乳腺癌转移
组蛋白赖氨酸特异性去甲基化酶 1 (LSD1) 在乳腺癌中过度活跃,这与肿瘤的转移有关。目前不可逆的 LSD1 抑制剂都是通过与黄素腺嘌呤二核苷酸辅因子共价结合合成的,由于对多种靶标的高亲和力,往往有副作用。在这里,我们鉴定了异连翘苷 (IFA),一种从连翘中分离的天然苯丙烷苷,作为 LSD1 的新型共价抑制剂。靶配体钓鱼技术和 LC-MS/MS 分析发现,IFA 可以通过 α,β-不饱和酮部分与 LSD1 的 Ser817 残基共价结合,以阻断 LSD1 的胺氧化酶样结构域。此外,IFA 处理后激活 LSD1 的下游信号通路 RBMS3/Twist1/MMP2,抑制 MDA-MB-231 细胞在体外和体内的转移。本研究为开发 LSD1 共价结合抑制剂提供了新的分子模板,为开发靶向 LSD1 的乳腺癌转移药物奠定了基础。
更新日期:2024-11-05
中文翻译:

异连翘苷作为组蛋白赖氨酸特异性去甲基化酶 1 共价抑制剂的靶向配体分离和鉴定,对抗乳腺癌转移
组蛋白赖氨酸特异性去甲基化酶 1 (LSD1) 在乳腺癌中过度活跃,这与肿瘤的转移有关。目前不可逆的 LSD1 抑制剂都是通过与黄素腺嘌呤二核苷酸辅因子共价结合合成的,由于对多种靶标的高亲和力,往往有副作用。在这里,我们鉴定了异连翘苷 (IFA),一种从连翘中分离的天然苯丙烷苷,作为 LSD1 的新型共价抑制剂。靶配体钓鱼技术和 LC-MS/MS 分析发现,IFA 可以通过 α,β-不饱和酮部分与 LSD1 的 Ser817 残基共价结合,以阻断 LSD1 的胺氧化酶样结构域。此外,IFA 处理后激活 LSD1 的下游信号通路 RBMS3/Twist1/MMP2,抑制 MDA-MB-231 细胞在体外和体内的转移。本研究为开发 LSD1 共价结合抑制剂提供了新的分子模板,为开发靶向 LSD1 的乳腺癌转移药物奠定了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号