当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Solvent/Protein Region Occupation of the Non-Nucleoside Reverse Transcriptase Inhibitor Binding Pocket for Improved Broad-Spectrum Anti-HIV-1 Efficacy: from Rigid Phenyl-Diarylpyrimidines to Flexible Hydrophilic Piperidine-Diarylpyrimidines
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.jmedchem.4c02413 Wen-Juan Huang, Christophe Pannecouque, Erik De Clercq, Angela Corona, Stefania Maloccu, Enzo Tramontano, Shuai Wang, Fen-Er Chen
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.jmedchem.4c02413 Wen-Juan Huang, Christophe Pannecouque, Erik De Clercq, Angela Corona, Stefania Maloccu, Enzo Tramontano, Shuai Wang, Fen-Er Chen
|
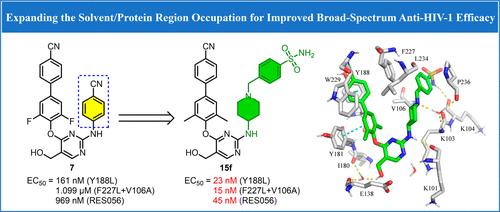
Considering the nonideal antiresistance efficacy of our previously reported non-nucleoside reverse transcriptase inhibitor 7, a series of novel piperidine-diarylpyrimidine derivatives were designed through expanding solvent/protein region occupation. The representative compound 15f proved to be exceptionally potent against Y188L (EC50 = 23 nM), F227L + V106A (EC50 = 15 nM) and RES056 (EC50 = 45 nM), significantly better than 7. This analog exerted strong inhibition against wild-type HIV-1 (EC50 = 3 nM) and single mutant strains (L100I, K103N, Y181C, E138 K). Notably, its cytotoxicity and selectivity (CC50 = 18.23 μM, SI = 6537) were 4-fold better than etravirine and rilpivirine. Additionally, it exhibited minimal suppression of CYP isoenzymes and hERG, indicating low potential for drug–drug interactions and cardiotoxicity. No significant acute toxicity and tissue damage at a dose of 2 g/kg were revealed. These findings lay the groundwork for the advancement of 15f as a highly potent, safe, and broad-spectrum NNRTI for HIV therapy.
中文翻译:

扩大非核苷逆转录酶抑制剂结合口袋的溶剂/蛋白质区域占据,以提高广谱抗 HIV-1 疗效:从刚性苯基-二芳基嘧啶到柔性亲水性哌啶-二芳基嘧啶
考虑到我们之前报道的非核苷逆转录酶抑制剂 7 的非理想抗耐药效果,通过扩大溶剂/蛋白质区域的占据设计了一系列新型哌啶-二芳基嘧啶衍生物。代表性化合物 15f 被证明对 Y188L (EC50 = 23 nM)、F227L + V106A (EC50 = 15 nM) 和 RES056 (EC50 = 45 nM) 非常有效,显著优于 7。该类似物对野生型 HIV-1 (EC50 = 3 nM) 和单个突变菌株 (L100I、K103N、Y181C、E138 K) 具有很强的抑制作用。值得注意的是,其细胞毒性和选择性 (CC50 = 18.23 μM,SI = 6537) 比依曲韦林和利匹韦林好 4 倍。此外,它对 CYP 同工酶和 hERG 的抑制最小,表明药物相互作用和心脏毒性的可能性较低。在 2 g/kg 剂量下未发现明显的急性毒性和组织损伤。这些发现为 15f 作为一种高效、安全和广谱的 HIV 治疗 NNRTI 奠定了基础。
更新日期:2024-11-05
中文翻译:

扩大非核苷逆转录酶抑制剂结合口袋的溶剂/蛋白质区域占据,以提高广谱抗 HIV-1 疗效:从刚性苯基-二芳基嘧啶到柔性亲水性哌啶-二芳基嘧啶
考虑到我们之前报道的非核苷逆转录酶抑制剂 7 的非理想抗耐药效果,通过扩大溶剂/蛋白质区域的占据设计了一系列新型哌啶-二芳基嘧啶衍生物。代表性化合物 15f 被证明对 Y188L (EC50 = 23 nM)、F227L + V106A (EC50 = 15 nM) 和 RES056 (EC50 = 45 nM) 非常有效,显著优于 7。该类似物对野生型 HIV-1 (EC50 = 3 nM) 和单个突变菌株 (L100I、K103N、Y181C、E138 K) 具有很强的抑制作用。值得注意的是,其细胞毒性和选择性 (CC50 = 18.23 μM,SI = 6537) 比依曲韦林和利匹韦林好 4 倍。此外,它对 CYP 同工酶和 hERG 的抑制最小,表明药物相互作用和心脏毒性的可能性较低。在 2 g/kg 剂量下未发现明显的急性毒性和组织损伤。这些发现为 15f 作为一种高效、安全和广谱的 HIV 治疗 NNRTI 奠定了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号