当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Morphological impact of 1-dimensional to 3-dimensional manganese dioxides on catalytic ozone decomposition correlated with crystal facets and lattice oxygen mobilities
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4en00857j Haotian Wu, Runduo Zhang, Bin Kang, Xiaonan Guo, Zhaoying Di, Kun Wang, Jingbo Jia, Ying Wei, Zhou-jun Wang
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4en00857j Haotian Wu, Runduo Zhang, Bin Kang, Xiaonan Guo, Zhaoying Di, Kun Wang, Jingbo Jia, Ying Wei, Zhou-jun Wang
|
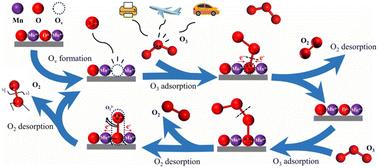
Ozone is a pollutant that has received widespread attention in recent years, and manganese dioxide (MnO2) has been widely used for catalytic ozone decomposition. However, few studies have described the structure–activity correlation of different morphological types of MnO2. In this study, a series of MnO2 crystals (α-, β-, γ-, δ-, ε- and λ-MnO2) were synthesized, and their catalytic activities on ozone decomposition (25 °C, dry air) were comparatively studied, which exhibited the order ε-MnO2 > α-MnO2 > γ-MnO2 > β-MnO2 ≈ δ-MnO2 > λ-MnO2. XRD and HRTEM results confirmed their diversities on the exposed crystal planes. It was confirmed that ε-MnO2 with the (1 0 2) plane had the largest number of oxygen vacancies and the best oxygen mobility. These findings elucidated the favorable performance of ε-MnO2 in the aforementioned tests. DFT calculations revealed the reaction mechanism, showing that ε-MnO2 has the lowest energy barrier for the rate-determining O22− desorption step (2.04 eV). This work illustrated the crucial role of oxygen vacancies and the mobility of lattice oxygen, shedding light on the strategies for rational design and control synthesis of effective catalysts for ozone elimination.
中文翻译:

一维至三维二氧化锰对催化臭氧分解的形态影响,与晶面和晶格氧迁移率相关
臭氧是近年来受到广泛关注的污染物,二氧化锰 (MnO2) 已被广泛用于催化臭氧分解。然而,很少有研究描述不同形态类型的 MnO2 的结构-活性相关性。本研究合成了一系列 MnO2 晶体(α-、β-、γ-、δ-、ε-和 λ-MnO2),并比较研究了它们对臭氧分解(25 °C,干燥空气)的催化活性,其顺序为 ε-MnO2 > α-MnO2 > γ-MnO2 > β-MnO2 ≈ δ-MnO2 > λ-MnO2.XRD 和 HRTEM 结果证实了它们在暴露的晶体面上的多样性。证实 (1 0 2) 平面的 ε-MnO2 具有最多的氧空位和最好的氧迁移率。这些发现阐明了 ε-MnO2 在上述测试中的良好性能。DFT 计算揭示了反应机理,表明 ε-MnO2 在确定速率的 O22− 解吸步骤 (2.04 eV) 中具有最低的能垒。这项工作说明了氧空位和晶格氧迁移率的关键作用,阐明了合理设计和控制合成有效臭氧消除催化剂的策略。
更新日期:2024-11-05
中文翻译:

一维至三维二氧化锰对催化臭氧分解的形态影响,与晶面和晶格氧迁移率相关
臭氧是近年来受到广泛关注的污染物,二氧化锰 (MnO2) 已被广泛用于催化臭氧分解。然而,很少有研究描述不同形态类型的 MnO2 的结构-活性相关性。本研究合成了一系列 MnO2 晶体(α-、β-、γ-、δ-、ε-和 λ-MnO2),并比较研究了它们对臭氧分解(25 °C,干燥空气)的催化活性,其顺序为 ε-MnO2 > α-MnO2 > γ-MnO2 > β-MnO2 ≈ δ-MnO2 > λ-MnO2.XRD 和 HRTEM 结果证实了它们在暴露的晶体面上的多样性。证实 (1 0 2) 平面的 ε-MnO2 具有最多的氧空位和最好的氧迁移率。这些发现阐明了 ε-MnO2 在上述测试中的良好性能。DFT 计算揭示了反应机理,表明 ε-MnO2 在确定速率的 O22− 解吸步骤 (2.04 eV) 中具有最低的能垒。这项工作说明了氧空位和晶格氧迁移率的关键作用,阐明了合理设计和控制合成有效臭氧消除催化剂的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号