当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tissue nano-transfection of antimicrobial genes drives bacterial biofilm killing in wounds and is potentially mediated by extracellular vesicles
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.jconrel.2024.10.071 Tatiana Z. Cuellar-Gaviria, Maria Angelica Rincon-Benavides, Hatice Nur Halipci Topsakal, Ana Isabel Salazar-Puerta, Shara Jaramillo-Garrido, Mia Kordowski, Carlos A. Vasquez-Martinez, Kim Truc Nguyen, Xilal Y. Rima, Pranav S.J.B. Rana, Orlando Combita-Heredia, Binbin Deng, Kavya Dathathreya, David W. McComb, Eduardo Reategui, Daniel Wozniak, Natalia Higuita-Castro, Daniel Gallego-Perez
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.jconrel.2024.10.071 Tatiana Z. Cuellar-Gaviria, Maria Angelica Rincon-Benavides, Hatice Nur Halipci Topsakal, Ana Isabel Salazar-Puerta, Shara Jaramillo-Garrido, Mia Kordowski, Carlos A. Vasquez-Martinez, Kim Truc Nguyen, Xilal Y. Rima, Pranav S.J.B. Rana, Orlando Combita-Heredia, Binbin Deng, Kavya Dathathreya, David W. McComb, Eduardo Reategui, Daniel Wozniak, Natalia Higuita-Castro, Daniel Gallego-Perez
|
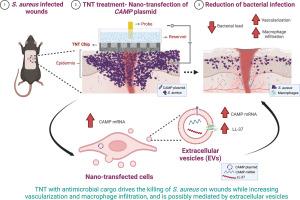
The emergence of bacteria that are resistant to antibiotics is on track to become a major global health crisis. Therefore, there is an urgent need for new treatment options. Here, we studied the implementation of tissue-nanotransfection (TNT) to treat Staphylococcus aureus -infected wounds by delivering gene cargos that boost the levels of naturally produced antimicrobial peptides. The Cathelicidin Antimicrobial Peptide gene (CAMP ), which produces the antimicrobial peptide LL-37, was used as model gene cargo. In vitro evaluation showed successful transfection and an increase in the transcription and translation of CAMP -coding plasmid in mouse primary epithelial cells. Moreover, we found that the extracellular vesicles (EVs) derived from the transfected cells (in vitro and in vivo) carried significantly higher concentrations of CAMP transcripts and LL-37 peptide compared to control EVs, possibly mediating the trafficking of the antimicrobial contents to other neighboring cells. The TNT platform was then used in vivo on an excisional wound model in mice to nanotransfect the CAMP -coding plasmid on the edge of infected wounds. After 4 days of daily treatment, we observed a significant decrease in the bacterial load in the CAMP -treated group compared to the sham group. Moreover, histological analysis and bacterial load quantification also revealed that TNT of CAMP on S. aureus -infected wounds was effective in treating biofilm progression by reducing the bacterial load. Lastly, we observed a significant increase in macrophage recruitment to the infected tissue, a robust increase in vascularization, as well as and an increased expression of IL10 and Fli1 . Our results demonstrate that TNT-based delivery of gene cargos coding for antimicrobial compounds to the wound is a promising approach for combating biofilm infections in wounds.
中文翻译:

抗菌基因的组织纳米转染驱动伤口中的细菌生物膜杀伤,并可能由细胞外囊泡介导
对抗生素具有耐药性的细菌的出现有望成为一场重大的全球健康危机。因此,迫切需要新的治疗方案。在这里,我们研究了组织纳米转染 (TNT) 的实施,通过递送提高天然产生的抗菌肽水平的基因货物来治疗金黄色葡萄球菌感染的伤口。产生抗菌肽 LL-37 的 Cathelicidin 抗菌肽基因 (CAMP) 被用作模型基因货物。体外评估显示小鼠原代上皮细胞中 CAMP 编码质粒的转染成功和转录和翻译增加。此外,我们发现与对照 EV 相比,来自转染细胞(体外和体内)衍生的细胞外囊泡 (EV) 携带浓度显着更高的 CAMP 转录本和 LL-37 肽,可能介导抗菌物质转运到其他邻近细胞。然后将 TNT 平台在小鼠的切除伤口模型上体内使用,以纳米转染感染伤口边缘的 CAMP 编码质粒。每日治疗 4 天后,我们观察到与假手术组相比,CAMP 治疗组的细菌载量显着降低。此外,组织学分析和细菌载量定量还显示,CAMP 对金黄色葡萄球菌感染伤口的 TNT 通过减少细菌载量有效治疗生物膜进展。最后,我们观察到巨噬细胞向感染组织的募集显着增加,血管形成的强劲增加,以及 IL10 和 Fli1 的表达增加。 我们的结果表明,基于 TNT 的编码抗菌化合物的基因货物递送到伤口是对抗伤口生物膜感染的一种很有前途的方法。
更新日期:2024-11-13
中文翻译:

抗菌基因的组织纳米转染驱动伤口中的细菌生物膜杀伤,并可能由细胞外囊泡介导
对抗生素具有耐药性的细菌的出现有望成为一场重大的全球健康危机。因此,迫切需要新的治疗方案。在这里,我们研究了组织纳米转染 (TNT) 的实施,通过递送提高天然产生的抗菌肽水平的基因货物来治疗金黄色葡萄球菌感染的伤口。产生抗菌肽 LL-37 的 Cathelicidin 抗菌肽基因 (CAMP) 被用作模型基因货物。体外评估显示小鼠原代上皮细胞中 CAMP 编码质粒的转染成功和转录和翻译增加。此外,我们发现与对照 EV 相比,来自转染细胞(体外和体内)衍生的细胞外囊泡 (EV) 携带浓度显着更高的 CAMP 转录本和 LL-37 肽,可能介导抗菌物质转运到其他邻近细胞。然后将 TNT 平台在小鼠的切除伤口模型上体内使用,以纳米转染感染伤口边缘的 CAMP 编码质粒。每日治疗 4 天后,我们观察到与假手术组相比,CAMP 治疗组的细菌载量显着降低。此外,组织学分析和细菌载量定量还显示,CAMP 对金黄色葡萄球菌感染伤口的 TNT 通过减少细菌载量有效治疗生物膜进展。最后,我们观察到巨噬细胞向感染组织的募集显着增加,血管形成的强劲增加,以及 IL10 和 Fli1 的表达增加。 我们的结果表明,基于 TNT 的编码抗菌化合物的基因货物递送到伤口是对抗伤口生物膜感染的一种很有前途的方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号