当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-selectivity relationship of anion exchange resins with different quaternary amine functional groups for highly selective removal of PFAS from chromium-plating wastewater
Water Research ( IF 11.4 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.watres.2024.122749 Xiangzhe Jiang, Yihua Luo, Shuang Mu, Bojiang Meng, Wei Wang, Gang Yu, Shubo Deng
Water Research ( IF 11.4 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.watres.2024.122749 Xiangzhe Jiang, Yihua Luo, Shuang Mu, Bojiang Meng, Wei Wang, Gang Yu, Shubo Deng
|
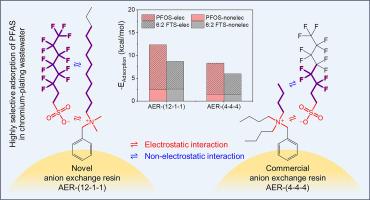
Anion exchange resin (AER) adsorption is an effective technology for the removal of per- and polyfluoroalkyl substances (PFAS) from wastewater. However, existing AERs with tributylamine functional groups have poor adsorption selectivity for perfluorinated carboxylic acids (PFCAs) and 6:2 fluorotelomer sulfonate (6:2 FTS), and the structure-selectivity relationship is still unclear. In this study, several novel gel AERs with long-chain amine groups were prepared. It was found that their adsorption selectivity for 6:2 FTS was 3.3-5.1 times that of commercial AERs, and the adsorption amount for 6:2 FTS in chromium-plating wastewater was 2.1 times that of the commercial PFA694E. On this basis, we synthesized 16 AERs with different quaternary amine functional groups, and explored the structure-selectivity relationship through the selectivity coefficients and adsorption energies of different AERs for seven typical PFAS. The order of adsorption selectivity of AERs with different quaternary amine groups for PFAS was AER-(12-1-1) > AER-(8-1-1) > AER-(4-4-4) > AER-(4-1-1) ≈ AER-(2-2-2) > AER-(1-1-1), where the three numbers are the carbon-chain lengths of the three alkyl groups attached to the nitrogen atom of the quaternary amine group. Density functional theory (DFT) calculations confirmed the enhanced adsorption selectivity and contribution of both non-electrostatic and electrostatic interactions by the long-chain amine groups, and a quantitative relationship between theoretical calculations and experimental results was established. These results could provide guidance for the development of efficient adsorbents for PFAS removal from wastewater.
中文翻译:

具有不同季胺官能团的阴离子交换树脂在镀铬废水中高选择性去除 PFAS 的结构-选择性关系
阴离子交换树脂 (AER) 吸附是一种从废水中去除全氟烷基和多氟烷基物质 (PFAS) 的有效技术。然而,现有的具有三丁胺官能团的 AER 对全氟羧酸 (PFCAs) 和 6:2 氟调聚物磺酸盐 (6:2 FTS) 的吸附选择性较差,结构-选择性关系尚不清楚。在这项研究中,制备了几种具有长链胺基的新型凝胶 AAR。结果发现,它们对 6:2 FTS 的吸附选择性是商用 AER 的 3.3-5.1 倍,6:2 FTS 在镀铬废水中的吸附量是商用 PFA694E 的 2.1 倍。在此基础上,我们合成了 16 种具有不同季胺官能团的 AAR,并通过不同 AER 的选择性系数和吸附能探索了 7 种典型 PFAS 的结构-选择性关系。不同季胺基团的AERs对PFAS的吸附选择性顺序为AER-(12-1-1) > AER-(8-1-1) > AER-(4-4-4) > AER-(4-1-1) ≈ AER-(2-2-2) > AER-(1-1-1),其中三个数字是季胺基氮原子上连接的三个烷基的碳链长度。密度泛函理论 (DFT) 计算证实了长链胺基团增强的吸附选择性和非静电和静电相互作用的贡献,并建立了理论计算和实验结果之间的定量关系。这些结果可为开发用于从废水中去除 PFAS 的高效吸附剂提供指导。
更新日期:2024-11-04
中文翻译:

具有不同季胺官能团的阴离子交换树脂在镀铬废水中高选择性去除 PFAS 的结构-选择性关系
阴离子交换树脂 (AER) 吸附是一种从废水中去除全氟烷基和多氟烷基物质 (PFAS) 的有效技术。然而,现有的具有三丁胺官能团的 AER 对全氟羧酸 (PFCAs) 和 6:2 氟调聚物磺酸盐 (6:2 FTS) 的吸附选择性较差,结构-选择性关系尚不清楚。在这项研究中,制备了几种具有长链胺基的新型凝胶 AAR。结果发现,它们对 6:2 FTS 的吸附选择性是商用 AER 的 3.3-5.1 倍,6:2 FTS 在镀铬废水中的吸附量是商用 PFA694E 的 2.1 倍。在此基础上,我们合成了 16 种具有不同季胺官能团的 AAR,并通过不同 AER 的选择性系数和吸附能探索了 7 种典型 PFAS 的结构-选择性关系。不同季胺基团的AERs对PFAS的吸附选择性顺序为AER-(12-1-1) > AER-(8-1-1) > AER-(4-4-4) > AER-(4-1-1) ≈ AER-(2-2-2) > AER-(1-1-1),其中三个数字是季胺基氮原子上连接的三个烷基的碳链长度。密度泛函理论 (DFT) 计算证实了长链胺基团增强的吸附选择性和非静电和静电相互作用的贡献,并建立了理论计算和实验结果之间的定量关系。这些结果可为开发用于从废水中去除 PFAS 的高效吸附剂提供指导。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号