当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical-Driven Amyloid Clearance for Therapeutics and Diagnostics of Alzheimer’s Disease
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.accounts.4c00458 Hye Yun Kim, YoungSoo Kim
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.accounts.4c00458 Hye Yun Kim, YoungSoo Kim
|
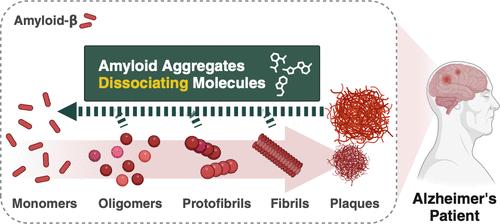
A century ago, German neurologist Alois Alzheimer documented the first case of Alzheimer’s disease (AD), illuminating cognitive impairments associated with the presence of abnormal protein clusters, including amyloid plaques and tau tangles, within the brain. In a typical physiological state, the equilibrium of amyloid-β (Aβ) levels is maintained, but aging can precipitate disruptions in the homeostasis of Aβ due to its overproduction, impaired clearance, and other factors, ultimately leading to its accumulation. Although the link between Aβ aggregates and neurodegeneration has long made Aβ a promising target for AD, decades without successful drug development targeting Aβ have generated skepticism regarding the efficacy of this strategy for AD therapy. However, recent approvals of anti-Aβ antibody drugs by the FDA, including aducanumab (Aduhelm), lecanemab (Leqembi), and donanemab (Kisunla), have prompted a re-evaluation of this perspective. These therapies have demonstrated efficacy in reducing brain Aβ levels, thereby decelerating disease progression and reaffirming Aβ as a key target. Despite advancements, immunotherapies are accompanied by considerable disadvantages, including adverse effects, high costs, and cumbersome administration. To address these limitations, our research has focused on developing small molecules that can mitigate the challenges of antibody treatments while offering practical and accessible options. We identified 4-(2-hydroxyethyl)-1-piperazine propanesulfonic acid (EPPS) as a promising compound that significantly reduces aggregated Aβ in the brain and enhances behavior in AD rodent models. Following administration, EPPS penetrates the blood-brain barrier (BBB) and binds to toxic Aβ aggregates, subsequently breaking them down into nontoxic monomers. This leads to two significant outcomes: a reduction of Aβ aggregates in the brain and a subsequent increase in Aβ monomers in blood. The monomeric Aβ, unlike its aggregated form, can now traverse the BBB and enter the bloodstream. This mechanism provides an innovative approach to AD treatment and diagnosis. By detaching cerebral Aβ aggregates, EPPS facilitates Aβ clearance and addresses a key pathological feature of AD. Concurrently, the increase in blood Aβ levels offers a potential biomarker for monitoring treatment efficacy and disease progression, thereby revolutionizing both AD treatment and diagnosis. Investigating the detailed mode of action of drug candidates requires structural information about a target protein. Unfortunately, the unstable and heterogeneous nature of Aβ aggregates, which form larger clusters, complicates the identification of these structures. Therefore, we developed new tools for screening small molecules by immobilizing monomeric Aβ and its fragments on plates. This allows us not only to identify novel compounds that target Aβ but also to elucidate their mechanisms of action, enabling the development of Aβ-targeting therapeutic avenues in AD. We believe that our work on chemical-driven amyloid clearance through small molecules represents an advance in AD research, offering chemical diversity and straightforward, economical development processes. In clinical practice, we anticipate that these findings will contribute to the development of patient-friendly therapeutic and diagnostic interventions, including self-administered and orally available options, thereby enhancing disease management and overall quality of life for individuals with AD. Furthermore, this research extends beyond AD, potentially offering insights into other neurodegenerative diseases characterized by protein aggregation.
中文翻译:

化学驱动的淀粉样蛋白清除率用于阿尔茨海默病的治疗和诊断
一个世纪前,德国神经学家阿洛伊斯·阿尔茨海默 (Alois Alzheimer) 记录了第一例阿尔茨海默病 (AD) 病例,阐明了与大脑内异常蛋白质簇(包括淀粉样蛋白斑块和 tau 缠结)相关的认知障碍。在典型的生理状态下,淀粉样蛋白-β (Aβ) 水平的平衡得以维持,但由于 Aβ 的过量产生、清除受损和其他因素,衰老会促使 Aβ 的体内平衡中断,最终导致其积累。尽管 Aβ 聚集体与神经退行性变之间的联系长期以来使 Aβ 成为 AD 的一个有前途的靶点,但几十年来没有成功开发针对 Aβ 的药物,这引起了人们对这种策略对 AD 治疗疗效的怀疑。然而,FDA 最近批准了抗 Aβ 抗体药物,包括 aducanumab (Aduhelm)、lecanemab (Leqembi) 和 donanemab (Kisunla),促使对这一观点进行了重新评估。这些疗法已被证明可有效降低脑 Aβ 水平,从而减缓疾病进展并重申 Aβ 是关键靶点。尽管取得了进步,但免疫疗法也伴随着相当大的缺点,包括不良反应、高成本和繁琐的管理。为了解决这些限制,我们的研究重点是开发可以减轻抗体治疗挑战的小分子,同时提供实用且可及的选择。我们确定 4-(2-羟乙基)-1-哌嗪丙磺酸 (EPPS) 是一种很有前途的化合物,可显着降低大脑中聚集的 Aβ 并增强 AD 啮齿动物模型的行为。 给药后,EPPS 穿透血脑屏障 (BBB) 并与有毒的 Aβ 聚集体结合,随后将它们分解成无毒的单体。这导致了两个重要的结果:大脑中 Aβ 聚集体的减少和血液中 Aβ 单体的增加。与其聚集形式不同,单体 Aβ 现在可以穿过 BBB 并进入血液。这种机制为 AD 治疗和诊断提供了一种创新的方法。通过分离脑 Aβ 聚集体,EPPS 促进 Aβ 清除并解决 AD 的关键病理特征。同时,血液 Aβ 水平的增加为监测治疗效果和疾病进展提供了潜在的生物标志物,从而彻底改变了 AD 的治疗和诊断。研究候选药物的详细作用模式需要有关靶蛋白的结构信息。不幸的是,形成更大簇的 Aβ 聚集体的不稳定和异质性使这些结构的鉴定变得复杂。因此,我们开发了通过将单体 Aβ 及其片段固定在板上来筛选小分子的新工具。这不仅使我们能够识别靶向 Aβ 的新型化合物,还可以阐明它们的作用机制,从而能够开发靶向 Aβ 的 AD 治疗途径。我们相信,我们在通过小分子进行化学驱动的淀粉样蛋白清除方面的工作代表了 AD 研究的进步,提供了化学多样性和简单、经济的开发过程。 在临床实践中,我们预计这些发现将有助于开发对患者友好的治疗和诊断干预措施,包括自我管理和口服选择,从而加强 AD 患者的疾病管理和整体生活质量。此外,这项研究超越了 AD,可能为其他以蛋白质聚集为特征的神经退行性疾病提供见解。
更新日期:2024-11-04
中文翻译:

化学驱动的淀粉样蛋白清除率用于阿尔茨海默病的治疗和诊断
一个世纪前,德国神经学家阿洛伊斯·阿尔茨海默 (Alois Alzheimer) 记录了第一例阿尔茨海默病 (AD) 病例,阐明了与大脑内异常蛋白质簇(包括淀粉样蛋白斑块和 tau 缠结)相关的认知障碍。在典型的生理状态下,淀粉样蛋白-β (Aβ) 水平的平衡得以维持,但由于 Aβ 的过量产生、清除受损和其他因素,衰老会促使 Aβ 的体内平衡中断,最终导致其积累。尽管 Aβ 聚集体与神经退行性变之间的联系长期以来使 Aβ 成为 AD 的一个有前途的靶点,但几十年来没有成功开发针对 Aβ 的药物,这引起了人们对这种策略对 AD 治疗疗效的怀疑。然而,FDA 最近批准了抗 Aβ 抗体药物,包括 aducanumab (Aduhelm)、lecanemab (Leqembi) 和 donanemab (Kisunla),促使对这一观点进行了重新评估。这些疗法已被证明可有效降低脑 Aβ 水平,从而减缓疾病进展并重申 Aβ 是关键靶点。尽管取得了进步,但免疫疗法也伴随着相当大的缺点,包括不良反应、高成本和繁琐的管理。为了解决这些限制,我们的研究重点是开发可以减轻抗体治疗挑战的小分子,同时提供实用且可及的选择。我们确定 4-(2-羟乙基)-1-哌嗪丙磺酸 (EPPS) 是一种很有前途的化合物,可显着降低大脑中聚集的 Aβ 并增强 AD 啮齿动物模型的行为。 给药后,EPPS 穿透血脑屏障 (BBB) 并与有毒的 Aβ 聚集体结合,随后将它们分解成无毒的单体。这导致了两个重要的结果:大脑中 Aβ 聚集体的减少和血液中 Aβ 单体的增加。与其聚集形式不同,单体 Aβ 现在可以穿过 BBB 并进入血液。这种机制为 AD 治疗和诊断提供了一种创新的方法。通过分离脑 Aβ 聚集体,EPPS 促进 Aβ 清除并解决 AD 的关键病理特征。同时,血液 Aβ 水平的增加为监测治疗效果和疾病进展提供了潜在的生物标志物,从而彻底改变了 AD 的治疗和诊断。研究候选药物的详细作用模式需要有关靶蛋白的结构信息。不幸的是,形成更大簇的 Aβ 聚集体的不稳定和异质性使这些结构的鉴定变得复杂。因此,我们开发了通过将单体 Aβ 及其片段固定在板上来筛选小分子的新工具。这不仅使我们能够识别靶向 Aβ 的新型化合物,还可以阐明它们的作用机制,从而能够开发靶向 Aβ 的 AD 治疗途径。我们相信,我们在通过小分子进行化学驱动的淀粉样蛋白清除方面的工作代表了 AD 研究的进步,提供了化学多样性和简单、经济的开发过程。 在临床实践中,我们预计这些发现将有助于开发对患者友好的治疗和诊断干预措施,包括自我管理和口服选择,从而加强 AD 患者的疾病管理和整体生活质量。此外,这项研究超越了 AD,可能为其他以蛋白质聚集为特征的神经退行性疾病提供见解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号