当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Balancing layered ordering and lattice oxygen stability for electrochemically stable high-nickel layered cathode for lithium-ion batteries
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.ensm.2024.103884 Gogwon Choe, Eunseong Choi, Yiseul Yoo, Kyung Yoon Chung, Hee-Dae Lim, Jaesub Kwon, Jaeik Kwak, Sang-Hoon You, Jong-Il Park, Sang Cheol Nam, Kyu-Young Park, Yong-Tae Kim
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.ensm.2024.103884 Gogwon Choe, Eunseong Choi, Yiseul Yoo, Kyung Yoon Chung, Hee-Dae Lim, Jaesub Kwon, Jaeik Kwak, Sang-Hoon You, Jong-Il Park, Sang Cheol Nam, Kyu-Young Park, Yong-Tae Kim
|
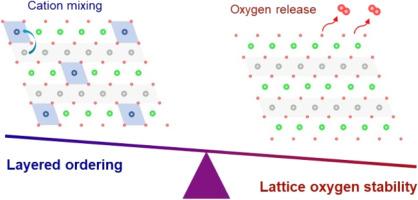
Despite the high-capacity nature of high-nickel cathode materials, achieving their practical implementation is challenging due to the susceptibility of atomic arrangement to calcining conditions. Extensive studies have enlightened the correlation between layered ordering and calcining conditions; nevertheless, the alterations in the electronic structure of lattice oxygen remain obscure. In this study, by comparing cathode materials with varying degrees of layered ordering, achieved through adjustments in calcination temperature and lithium equivalent, it is shown that although layered ordering increases, it compromises the electronic structure, creating a labile lattice oxygen environment. Fine structural analysis reveals that a higher local Li/O ratio in highly ordered cathode subsequently alters the band structure by narrowing the band gap between Ni 3d and O 2p , which enhances transition metal–oxygen covalency, and reduces the oxygen vacancy formation energy, adversely affecting cyclability. In highly ordered cathode, the tendency of lattice oxygen to reside within a Li-enriched environment arises from the changes in the favorability of non-paired antisite defects contingent upon the calcination temperature and lithium equivalent. This research underscores the need to balance layered ordering and lattice oxygen stability, offering important insights for the future design of high-nickel cathodes.
中文翻译:

平衡分层有序和晶格氧稳定性,用于锂离子电池的电化学稳定性高镍层状阴极
尽管高镍正极材料具有高容量性质,但由于原子排列对煅烧条件的敏感性,实现其实际实施具有挑战性。广泛的研究阐明了分层排序和煅烧条件之间的相关性;然而,晶格氧的电子结构的变化仍然不清楚。在这项研究中,通过比较通过调整煅烧温度和锂当量实现的不同程度层状有序的正极材料,表明虽然层状有序增加,但它损害了电子结构,创造了不稳定的晶格氧环境。精细结构分析表明,在高度有序的阴极中,较高的局部 Li/O 比率随后通过缩小 Ni 3d 和 O 2p 之间的带隙来改变能带结构,从而增强过渡金属-氧共价,并降低氧空位形成能,从而对可循环性产生不利影响。在高度有序的阴极中,晶格氧驻留在富锂环境中的趋势是由取决于煅烧温度和锂当量的非成对反位缺陷的有利性变化引起的。这项研究强调了平衡分层有序和晶格氧稳定性的必要性,为未来高镍阴极的设计提供了重要的见解。
更新日期:2024-11-04
中文翻译:

平衡分层有序和晶格氧稳定性,用于锂离子电池的电化学稳定性高镍层状阴极
尽管高镍正极材料具有高容量性质,但由于原子排列对煅烧条件的敏感性,实现其实际实施具有挑战性。广泛的研究阐明了分层排序和煅烧条件之间的相关性;然而,晶格氧的电子结构的变化仍然不清楚。在这项研究中,通过比较通过调整煅烧温度和锂当量实现的不同程度层状有序的正极材料,表明虽然层状有序增加,但它损害了电子结构,创造了不稳定的晶格氧环境。精细结构分析表明,在高度有序的阴极中,较高的局部 Li/O 比率随后通过缩小 Ni 3d 和 O 2p 之间的带隙来改变能带结构,从而增强过渡金属-氧共价,并降低氧空位形成能,从而对可循环性产生不利影响。在高度有序的阴极中,晶格氧驻留在富锂环境中的趋势是由取决于煅烧温度和锂当量的非成对反位缺陷的有利性变化引起的。这项研究强调了平衡分层有序和晶格氧稳定性的必要性,为未来高镍阴极的设计提供了重要的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号