当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Cholesterol in M2 Clustering and Viral Budding Explained
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.jctc.4c01026 Dimitrios Kolokouris, Iris E. Kalenderoglou, Anna L. Duncan, Robin A. Corey, Mark S. P. Sansom, Antonios Kolocouris
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.jctc.4c01026 Dimitrios Kolokouris, Iris E. Kalenderoglou, Anna L. Duncan, Robin A. Corey, Mark S. P. Sansom, Antonios Kolocouris
|
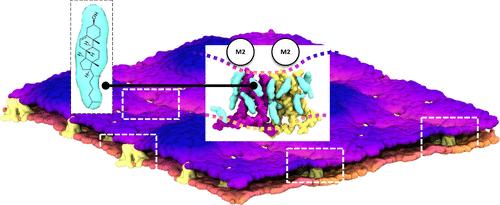
The influenza A M2 homotetrameric channel consists of four transmembrane (TM) and four amphipathic helices (AHs). This viral proton channel is suggested to form clusters in the catenoid budding neck areas in raft-like domains of the plasma membrane, resulting in cell membrane scission and viral release. The channel clustering environment is rich in cholesterol. Previous experiments have shown that cholesterol significantly contributes to lipid bilayer undulations in viral buds. However, a clear explanation of membrane curvature from the distribution of cholesterol around the M2TM-AH clusters is lacking. Using coarse-grained molecular dynamics simulations of M2TM-AH in bilayers, we observed that M2 channels form specific, C2-symmetric, clusters with conical shapes driven by the attraction of their AHs. We showed that cholesterol stabilized the formation of M2 channel clusters by filling and bridging the conical gap between M2 channels at specific sites in the N-termini of adjacent channels or via the C-terminal region of TM and AHs, with the latter sites displaying a longer interaction time and higher stability. The potential of mean force calculations showed that when cholesterols occupy the identified interfacial binding sites between two M2 channels, the dimer is stabilized by 11 kJ/mol. This translates to the cholesterol-bound dimer being populated by almost 2 orders of magnitude compared to a dimer lacking cholesterol. We demonstrated that the cholesterol-bridged M2 channels can exert a lateral force on the surrounding membrane to induce the necessary negative Gaussian curvature profile, which permits spontaneous scission of the catenoid membrane neck and leads to viral buds and scission.
中文翻译:

胆固醇在 M2 聚集和病毒出芽中的作用解释
甲型流感 M2 同源四聚体通道由四个跨膜 (TM) 和四个两亲性螺旋 (AH) 组成。这种病毒质子通道被认为在质膜的筏状结构域的链状出芽颈部区域形成簇,导致细胞膜分裂和病毒释放。通道聚类环境富含胆固醇。以前的实验表明,胆固醇显着导致病毒芽中的脂质双层波动。然而,缺乏从 M2TM-AH 簇周围胆固醇的分布中对膜曲率的明确解释。使用双层中 M2TM-AH 的粗粒度分子动力学模拟,我们观察到 M2 通道在其 AH 的吸引力驱动下形成特定的、C2 对称的圆锥形簇。我们发现,胆固醇通过在相邻通道的 N 末端的特定位点或通过 TM 和 AHs 的 C 端区域填充和桥接 M2 通道之间的锥形间隙来稳定 M2 通道簇的形成,后者位点显示出更长的相互作用时间和更高的稳定性。平均力计算的潜力表明,当胆固醇占据两个 M2 通道之间确定的界面结合位点时,二聚体稳定 11 kJ/mol。这意味着与缺乏胆固醇的二聚体相比,胆固醇结合的二聚体的填充量几乎高出 2 个数量级。我们证明胆固醇桥接的 M2 通道可以对周围的膜施加侧向力,以诱导必要的负高斯曲率剖面,这允许链状膜颈部自发断裂并导致病毒芽和断裂。
更新日期:2024-11-04
中文翻译:

胆固醇在 M2 聚集和病毒出芽中的作用解释
甲型流感 M2 同源四聚体通道由四个跨膜 (TM) 和四个两亲性螺旋 (AH) 组成。这种病毒质子通道被认为在质膜的筏状结构域的链状出芽颈部区域形成簇,导致细胞膜分裂和病毒释放。通道聚类环境富含胆固醇。以前的实验表明,胆固醇显着导致病毒芽中的脂质双层波动。然而,缺乏从 M2TM-AH 簇周围胆固醇的分布中对膜曲率的明确解释。使用双层中 M2TM-AH 的粗粒度分子动力学模拟,我们观察到 M2 通道在其 AH 的吸引力驱动下形成特定的、C2 对称的圆锥形簇。我们发现,胆固醇通过在相邻通道的 N 末端的特定位点或通过 TM 和 AHs 的 C 端区域填充和桥接 M2 通道之间的锥形间隙来稳定 M2 通道簇的形成,后者位点显示出更长的相互作用时间和更高的稳定性。平均力计算的潜力表明,当胆固醇占据两个 M2 通道之间确定的界面结合位点时,二聚体稳定 11 kJ/mol。这意味着与缺乏胆固醇的二聚体相比,胆固醇结合的二聚体的填充量几乎高出 2 个数量级。我们证明胆固醇桥接的 M2 通道可以对周围的膜施加侧向力,以诱导必要的负高斯曲率剖面,这允许链状膜颈部自发断裂并导致病毒芽和断裂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号