当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combustion Enhancement of Ammonia by Cofiring Oxymethylene Ethers (OMEn, n = 0–2): An Experimental and Kinetic Modeling Investigation
Energy & Fuels ( IF 5.2 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.energyfuels.4c03827 Bilal Hussain, Jun Fang, Jianguo Zhang, Wei Li, Yuyang Li
Energy & Fuels ( IF 5.2 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.energyfuels.4c03827 Bilal Hussain, Jun Fang, Jianguo Zhang, Wei Li, Yuyang Li
|
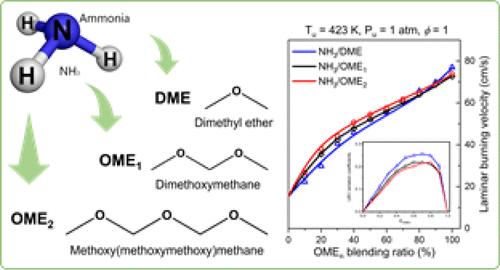
Ammonia (NH3) is a promising zero-carbon fuel with an exceptionally high hydrogen density. However, the feasibility of employing ammonia as a future fuel faces several obstacles including low combustion intensity. Co-firing reactive carbon-neutral fuels, such as oxymethylene ethers (OMEn) with NH3 emerges as an effective approach to enhance NH3 combustion. This work investigates the laminar flame propagation of NH3 cofired with dimethyl ether (DME), dimethoxymethane (OME1), and methoxy(methoxymethoxy)methane (OME2) using a high-pressure high-temperature constant-volume combustion vessel. Laminar burning velocities (LBVs) are measured at an initial temperature of 423 K and pressures of 1–10 atm. A kinetic model for NH3/OMEn combustion is developed and validated against the measured LBVs in this study, as well as LBVs and speciation data in literature. Both the experimental and kinetic modeling studies indicate the positive effect of cofiring of OMEn on ammonia combustion enhancement. The LBV levels of the NH3/OMEn mixture can be similar to that of methane. The effects of cofiring fuel compositions, equivalence ratios, and pressures are investigated using modeling analysis and the modified fictitious diluent gas method. In mixture combustion, the reaction pathways of ammonia, DME, OME1, and OME2 remain almost unchanged compared to single fuel combustion, despite the slight contribution of C–N interaction. Combustion enhancements result from both chemical effects and thermal effects and their contribution ratios vary according to equivalence ratios and fuel compositions. At ϕ = 1.6, the contribution of chemical effects increases in the order 50%NH3/50%DME, 50%NH3/50%OME1, and 50%NH3/50%OME2. Though there are similar LBVs for DME, OME1, and OME2, the mixture LBVs follow the sequence of 50%NH3/50%DME < 50%NH3/50%OME1 < 50%NH3/50%OME2, which can be attributed to the influence of their lower heating values. A quasi-square relationship between normalized LBVs and energy fraction can be derived by using the correction of the energy fraction for the three NH3/OMEn mixtures.
中文翻译:

通过共烧氧亚甲基醚增强氨的燃烧 (OMEn, n = 0–2):实验和动力学建模研究
氨 (NH3) 是一种很有前途的零碳燃料,具有极高的氢密度。然而,使用氨作为未来燃料的可行性面临几个障碍,包括燃烧强度低。将活性碳中和燃料,如氧亚甲基醚 (OMEn) 与 NH3 共燃烧,成为增强 NH3 燃烧的有效方法。本工作使用高压高温恒容燃烧容器研究了 NH3 与二甲醚 (DME)、二甲氧基甲烷 (OME1) 和甲氧基(甲氧基甲氧基)甲烷 (OME2) 共烧的层流火焰传播。层流燃烧速度 (LBV) 是在 423 K 的初始温度和 1-10 个大气压的压力下测量的。本研究开发了 NH3/OMEn 燃烧的动力学模型,并根据本研究中测量的 LBV 以及文献中的 LBV 和物种形成数据进行了验证。实验和动力学建模研究表明,OMEn 共烧对氨燃烧增强有积极影响。NH3/OMEn 混合物的 LBV 水平可能与甲烷相似。使用建模分析和改进的虚构稀释气体方法研究了混合燃烧燃料成分、等效比和压力的影响。在混合物燃烧中,氨、DME、OME1 和 OME2 的反应途径与单一燃料燃烧相比几乎没有变化,尽管 C-N 相互作用的贡献很小。燃烧增强是由化学效应和热效应引起的,它们的贡献比根据当量比和燃料成分而变化。φ = 1 时。6、化学效应的贡献按50%NH3/50%DME、50%NH3/50%OME1和50%NH3/50%OME2的顺序增加。尽管 DME、OME1 和 OME2 有类似的 LBV,但混合物 LBV 遵循 50%NH3/50%DME < 50%NH3/50%OME1 < 50%NH3/50%OME2 的顺序,这可以归因于它们较低的加热值的影响。归一化 LBV 和能量分数之间的准平方关系可以通过使用三种 NH3/OMEn 混合物的能量分数校正来得出。
更新日期:2024-11-04
中文翻译:

通过共烧氧亚甲基醚增强氨的燃烧 (OMEn, n = 0–2):实验和动力学建模研究
氨 (NH3) 是一种很有前途的零碳燃料,具有极高的氢密度。然而,使用氨作为未来燃料的可行性面临几个障碍,包括燃烧强度低。将活性碳中和燃料,如氧亚甲基醚 (OMEn) 与 NH3 共燃烧,成为增强 NH3 燃烧的有效方法。本工作使用高压高温恒容燃烧容器研究了 NH3 与二甲醚 (DME)、二甲氧基甲烷 (OME1) 和甲氧基(甲氧基甲氧基)甲烷 (OME2) 共烧的层流火焰传播。层流燃烧速度 (LBV) 是在 423 K 的初始温度和 1-10 个大气压的压力下测量的。本研究开发了 NH3/OMEn 燃烧的动力学模型,并根据本研究中测量的 LBV 以及文献中的 LBV 和物种形成数据进行了验证。实验和动力学建模研究表明,OMEn 共烧对氨燃烧增强有积极影响。NH3/OMEn 混合物的 LBV 水平可能与甲烷相似。使用建模分析和改进的虚构稀释气体方法研究了混合燃烧燃料成分、等效比和压力的影响。在混合物燃烧中,氨、DME、OME1 和 OME2 的反应途径与单一燃料燃烧相比几乎没有变化,尽管 C-N 相互作用的贡献很小。燃烧增强是由化学效应和热效应引起的,它们的贡献比根据当量比和燃料成分而变化。φ = 1 时。6、化学效应的贡献按50%NH3/50%DME、50%NH3/50%OME1和50%NH3/50%OME2的顺序增加。尽管 DME、OME1 和 OME2 有类似的 LBV,但混合物 LBV 遵循 50%NH3/50%DME < 50%NH3/50%OME1 < 50%NH3/50%OME2 的顺序,这可以归因于它们较低的加热值的影响。归一化 LBV 和能量分数之间的准平方关系可以通过使用三种 NH3/OMEn 混合物的能量分数校正来得出。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号