当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MOx catalysts
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.checat.2024.101167 Megumi Okazaki, Yasuomi Yamazaki, Daling Lu, Shunsuke Nozawa, Osamu Ishitani, Kazuhiko Maeda
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.checat.2024.101167 Megumi Okazaki, Yasuomi Yamazaki, Daling Lu, Shunsuke Nozawa, Osamu Ishitani, Kazuhiko Maeda
|
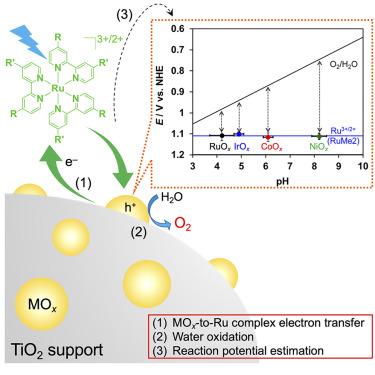
Photochemical water oxidation in the presence of a Ru(II) photosensitizer to form O2 is one of the most studied reactions in (photo)catalysis for both homogeneous and heterogeneous systems. In the present work, several Ru(II)-tris-diimine-type complexes with different ligands were used under a wide pH range (3.7–9.4) and over different transition-metal oxide (MOx) catalysts to reveal the factors that govern the O2 evolution activity. Most importantly, the results clarified that a certain “threshold” potential determines whether water oxidation can proceed and that this potential is related to the energy barrier for electron transfer from the MOx catalyst to the Ru(II) photosensitizer. The results of this work highlight that the potential of the electrons involved in the water oxidation on MOx catalysts can be estimated through the simple application of a photochemical reaction, which will be a useful measure for assessing the water oxidation activity of suspended nanoparticle catalysts.
中文翻译:

发现使用 Ru(II) 光敏剂和 MOx 催化剂触发光化学水氧化的阈值电位
在 Ru(II) 光敏剂存在下形成 O2 的光化学水氧化是均相和非均相体系的(光)催化中研究最多的反应之一。在本工作中,在较宽的 pH 范围 (3.7–9.4) 和不同的过渡金属氧化物 (MOx) 催化剂下使用几种具有不同配体的 Ru(II)-三三亚胺型络合物,以揭示控制 O2 析出活性的因素。最重要的是,结果阐明了一定的“阈值”电位决定了水氧化是否可以进行,并且该电位与电子从 MOx 催化剂转移到 Ru(II) 光敏剂的能垒有关。这项工作的结果强调,可以通过简单的光化学反应来估计 MOx 催化剂上参与水氧化的电子的电位,这将是评估悬浮纳米颗粒催化剂的水氧化活性的有用措施。
更新日期:2024-11-04
中文翻译:

发现使用 Ru(II) 光敏剂和 MOx 催化剂触发光化学水氧化的阈值电位
在 Ru(II) 光敏剂存在下形成 O2 的光化学水氧化是均相和非均相体系的(光)催化中研究最多的反应之一。在本工作中,在较宽的 pH 范围 (3.7–9.4) 和不同的过渡金属氧化物 (MOx) 催化剂下使用几种具有不同配体的 Ru(II)-三三亚胺型络合物,以揭示控制 O2 析出活性的因素。最重要的是,结果阐明了一定的“阈值”电位决定了水氧化是否可以进行,并且该电位与电子从 MOx 催化剂转移到 Ru(II) 光敏剂的能垒有关。这项工作的结果强调,可以通过简单的光化学反应来估计 MOx 催化剂上参与水氧化的电子的电位,这将是评估悬浮纳米颗粒催化剂的水氧化活性的有用措施。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号