当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micellar “Click” Nanoreactors: Spiking Pluronic-Based Micelles with Polymeric Ligands
Macromolecules ( IF 5.1 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.macromol.4c01425 Krishna Vippala, Shreyas Shankar Wagle, Parul Rathee, Keerthana Mulamukkil, Yousif Ayoub, Arthur Komlosh, Sharon Gazal, Bianca Avramovitch, Roey J. Amir
Macromolecules ( IF 5.1 ) Pub Date : 2024-11-04 , DOI: 10.1021/acs.macromol.4c01425 Krishna Vippala, Shreyas Shankar Wagle, Parul Rathee, Keerthana Mulamukkil, Yousif Ayoub, Arthur Komlosh, Sharon Gazal, Bianca Avramovitch, Roey J. Amir
|
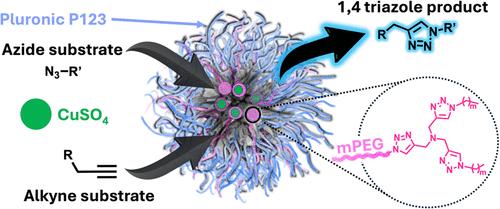
In recent years, the development of nanoreactors, such as micellar nanoreactors (MNRs) for catalytic transformations, has gained significant attention due to their potential in enhancing reaction rates, selectivity, efficiency, and, as importantly, the ability to conduct organic chemistry in aqueous solutions. Among these, the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction represents a pivotal transformation and is widely used in the synthesis of bioconjugates, pharmaceuticals, and advanced materials. This study aims toward advancing our understanding of the design and utilization of polymeric amphiphiles containing tris-triazole ligands as an integral element for CuAAC reactions within MNRs. Specifically, our investigation delves into three critical factors that influence the reaction rate within MNRs: hydrophobicity, architectural configuration of the polymeric ligands, and their concentration. Utilizing the high molecular precision of dendritic amphiphiles, we synthesized polymeric ligands with two distinct architectures, namely, PEG-ditris-triazole amphiphile (DTA) and PEG-monotris-triazole amphiphile (MTA), and explored their CuAAC reactivity through coassembly with commercially available Pluronic P123 amphiphiles. The results indicate that the architecture and the concentration of the polymeric ligands play more dominant roles in influencing the reaction rate than the hydrophobicity of the dendritic blocks. Notably, while MNRs assembled from solely DTA showed a dampened reaction rate, spiking P123 micelles with DTA yielded an MNR with significantly faster rates. Moreover, P123 MNRs spiked with the synthesized MTA demonstrated increased CuAAC reaction rates compared to those spiked with the DTA, and they even outperformed the widely used Tris(benzyltriazolylmethyl)amine ligand. These findings provide valuable insights into the design principles of polymer-based ligands for constructing reactive MNRs and other types of nanoreactors for efficient catalytic transformations.
中文翻译:

胶束 “Click” 纳米反应器:具有聚合物配体的加标 Pluronic 基胶束
近年来,纳米反应器(例如用于催化转化的胶束纳米反应器 (MNR))的开发因其在提高反应速率、选择性、效率以及同样重要的是在水溶液中进行有机化学的能力而受到广泛关注。其中,铜 (I) 催化的叠氮化物-炔烃环加成反应 (CuAAC) 代表了关键的转变,广泛用于生物偶联物、药物和先进材料的合成。本研究旨在推进我们对含有三三唑配体的聚合物两亲物的设计和利用的理解,这些聚合物是 MNR 内 CuAAC 反应的组成部分。具体来说,我们的研究深入探讨了影响 MNR 内反应速率的三个关键因素:疏水性、聚合物配体的结构构型及其浓度。利用树突状两亲物的高分子精度,我们合成了具有两种不同结构的聚合物配体,即 PEG-二三唑-两亲物 (DTA) 和 PEG-单三唑-两亲物 (MTA),并通过与市售的 Pluronic P123 两亲物共组装来探索它们的 CuAAC 反应性。结果表明,聚合物配体的结构和浓度在影响反应速率方面比树枝状嵌段的疏水性起着更主要的作用。值得注意的是,虽然仅由 DTA 组装的 MNR 显示出抑制的反应速率,但用 DTA 加标 P123 胶束会产生速率明显更快的 MNR。 此外,与加标 DTA 的 P123 MNR 相比,加标合成的 MTA 的 P123 MNR 表现出更高的 CuAAC 反应速率,它们甚至优于广泛使用的 Tris(苄基三唑基甲基)胺配体。这些发现为基于聚合物的配体的设计原理提供了有价值的见解,用于构建反应性 MNR 和其他类型的纳米反应器以实现高效催化转化。
更新日期:2024-11-04
中文翻译:

胶束 “Click” 纳米反应器:具有聚合物配体的加标 Pluronic 基胶束
近年来,纳米反应器(例如用于催化转化的胶束纳米反应器 (MNR))的开发因其在提高反应速率、选择性、效率以及同样重要的是在水溶液中进行有机化学的能力而受到广泛关注。其中,铜 (I) 催化的叠氮化物-炔烃环加成反应 (CuAAC) 代表了关键的转变,广泛用于生物偶联物、药物和先进材料的合成。本研究旨在推进我们对含有三三唑配体的聚合物两亲物的设计和利用的理解,这些聚合物是 MNR 内 CuAAC 反应的组成部分。具体来说,我们的研究深入探讨了影响 MNR 内反应速率的三个关键因素:疏水性、聚合物配体的结构构型及其浓度。利用树突状两亲物的高分子精度,我们合成了具有两种不同结构的聚合物配体,即 PEG-二三唑-两亲物 (DTA) 和 PEG-单三唑-两亲物 (MTA),并通过与市售的 Pluronic P123 两亲物共组装来探索它们的 CuAAC 反应性。结果表明,聚合物配体的结构和浓度在影响反应速率方面比树枝状嵌段的疏水性起着更主要的作用。值得注意的是,虽然仅由 DTA 组装的 MNR 显示出抑制的反应速率,但用 DTA 加标 P123 胶束会产生速率明显更快的 MNR。 此外,与加标 DTA 的 P123 MNR 相比,加标合成的 MTA 的 P123 MNR 表现出更高的 CuAAC 反应速率,它们甚至优于广泛使用的 Tris(苄基三唑基甲基)胺配体。这些发现为基于聚合物的配体的设计原理提供了有价值的见解,用于构建反应性 MNR 和其他类型的纳米反应器以实现高效催化转化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号