当前位置:
X-MOL 学术
›
Cem. Concr. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the direct carbonation process of Ca2SiO4 based on ReaxFF MD simulation and experiments
Cement and Concrete Research ( IF 10.9 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.cemconres.2024.107711
Ya-Jun Wang, Xiao-Pei Zhang, Dong-Mei Liu, Jun-Guo Li, Jian-Bao Zhang, Yu-Wei Zhang, Ya-Nan Zeng, Yi-Tong Wang, Bao Liu, Xi Zhang, Ya-Jing Zhang
Cement and Concrete Research ( IF 10.9 ) Pub Date : 2024-11-04 , DOI: 10.1016/j.cemconres.2024.107711
Ya-Jun Wang, Xiao-Pei Zhang, Dong-Mei Liu, Jun-Guo Li, Jian-Bao Zhang, Yu-Wei Zhang, Ya-Nan Zeng, Yi-Tong Wang, Bao Liu, Xi Zhang, Ya-Jing Zhang
|
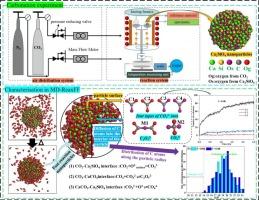
Ca2 SiO4 is the primary carbonation-reactive mineral in steel slag, and demonstrates significant carbon sequestration potential, yet its microscopic reaction processes remain unclear. This study investigated the carbonation behavior of Ca2 SiO4 using ReaxFF MD simulations. The results indicated that as CO2 concentration increased, the capture rate of Ca2 SiO4 decreased, and the molecular structure of the resulting CaCO3 varied in oxygen origin. At room temperature, the carbonation rate of Ca₂SiO₄ gradually decreased over time until it reached equilibrium. Increasing the temperature could reactivate the carbonation, but the rate would still decline until it reached equilibrium again. Higher temperatures could accelerate the formation of the intermediate C2 O5 2− and internal CO3 2− diffusion, thereby boosting the carbonation and increasing CO2 adsorption. This study investigated the carbonation of Ca2 SiO4 at the atomic level, aiming to link microscopic molecular processes with macroscopic experimental phenomena, thereby providing a theoretical foundation for enhancing the carbonation efficiency of steel slag.
中文翻译:

基于 ReaxFF MD 模拟和实验的 Ca2SiO4 直接碳化过程
Ca2SiO4 是钢渣中的主要碳化反应矿物,具有显著的固碳潜力,但其微观反应过程仍不清楚。本研究使用 ReaxFF MD 模拟研究了 Ca2SiO4 的碳化行为。结果表明,随着 CO2 浓度的增加,Ca2SiO4 的捕获率降低,所得 CaCO3 的分子结构因氧来源而异。在室温下,Ca₂SiO₄ 的碳化速率随着时间的推移逐渐降低,直到达到平衡。提高温度可以重新激活碳酸化,但速率仍会下降,直到再次达到平衡。较高的温度会加速中间体 C2O52− 和内部 CO32− 扩散的形成,从而促进碳化并增加 CO2 吸附。本研究在原子水平上研究了 Ca2SiO4 的碳化过程,旨在将微观分子过程与宏观实验现象联系起来,从而为提高钢渣的碳化效率提供理论基础。
更新日期:2024-11-04
中文翻译:

基于 ReaxFF MD 模拟和实验的 Ca2SiO4 直接碳化过程
Ca2SiO4 是钢渣中的主要碳化反应矿物,具有显著的固碳潜力,但其微观反应过程仍不清楚。本研究使用 ReaxFF MD 模拟研究了 Ca2SiO4 的碳化行为。结果表明,随着 CO2 浓度的增加,Ca2SiO4 的捕获率降低,所得 CaCO3 的分子结构因氧来源而异。在室温下,Ca₂SiO₄ 的碳化速率随着时间的推移逐渐降低,直到达到平衡。提高温度可以重新激活碳酸化,但速率仍会下降,直到再次达到平衡。较高的温度会加速中间体 C2O52− 和内部 CO32− 扩散的形成,从而促进碳化并增加 CO2 吸附。本研究在原子水平上研究了 Ca2SiO4 的碳化过程,旨在将微观分子过程与宏观实验现象联系起来,从而为提高钢渣的碳化效率提供理论基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号