当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling distinct bonding patterns in noble gas hydrides via interference energy analysis
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4cp04028g Lucas Araujo, Marco A. C. Nascimento, Thiago M. Cardozo, Felipe Fantuzzi
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4cp04028g Lucas Araujo, Marco A. C. Nascimento, Thiago M. Cardozo, Felipe Fantuzzi
|
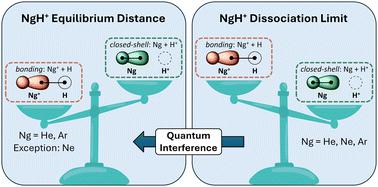
Despite their apparent simplicity, the helium hydride ion (HeH+) and its analogues with heavier noble gas (Ng) atoms present intriguing challenges due to their unusual electronic structures and distinct ground-state heterolytic bond dissociation profiles. In this work, we employ modern valence bond calculations and the interference energy analysis to investigate the nature of the chemical bond in NgH+ (Ng = He, Ne, Ar). Our findings reveal that the energy well formation in their ground-state potential energy curves is driven by a reduction in kinetic energy caused by quantum interference, identical to cases of homolytic bond dissociation. However, clear differences in bonding situation emerge: in HeH+ and ArH+, electron charge transfer leads to Ng+–H covalent bonds, while in NeH+, a preferred Ne + H+ valence bond structure suggests the formation of a dative bond. This study highlights the distinct bonding mechanisms within the NgH+ series, showcasing the interplay between quantum interference and quasi-classical effects in molecules featuring noble gases.
中文翻译:

通过干涉能量分析揭示惰性气体氢化物中独特的键合模式
尽管氦氢离子 (HeH+) 及其与较重的惰性气体 (Ng) 原子的类似物表面上很简单,但由于它们不寻常的电子结构和独特的基态异质解离键解离曲线,它们带来了有趣的挑战。在这项工作中,我们采用现代价键计算和干涉能分析来研究 NgH+ (Ng = He, Ne, Ar) 中化学键的性质。我们的研究结果表明,其基态势能曲线中的能量阱形成是由量子干涉引起的动能减少驱动的,这与同解键解离的情况相同。然而,键合情况出现了明显的差异:在 HeH+ 和 ArH+ 中,电子电荷转移导致 Ng+–H 共价键,而在 NeH+ 中,优选的 Ne + H+ 价键结构表明形成配格键。本研究强调了 NgH+ 系列中独特的键合机制,展示了具有惰性气体的分子中量子干涉和准经典效应之间的相互作用。
更新日期:2024-11-08
中文翻译:

通过干涉能量分析揭示惰性气体氢化物中独特的键合模式
尽管氦氢离子 (HeH+) 及其与较重的惰性气体 (Ng) 原子的类似物表面上很简单,但由于它们不寻常的电子结构和独特的基态异质解离键解离曲线,它们带来了有趣的挑战。在这项工作中,我们采用现代价键计算和干涉能分析来研究 NgH+ (Ng = He, Ne, Ar) 中化学键的性质。我们的研究结果表明,其基态势能曲线中的能量阱形成是由量子干涉引起的动能减少驱动的,这与同解键解离的情况相同。然而,键合情况出现了明显的差异:在 HeH+ 和 ArH+ 中,电子电荷转移导致 Ng+–H 共价键,而在 NeH+ 中,优选的 Ne + H+ 价键结构表明形成配格键。本研究强调了 NgH+ 系列中独特的键合机制,展示了具有惰性气体的分子中量子干涉和准经典效应之间的相互作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号