当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A combination of experimental and theoretical methods in evaluating triazole derivatives' mild steel corrosion inhibition ability in an acidic solution
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4cp03537b Ngo Hong Cat Van, Nguyen Quang Trung, Pham Dinh Tu Tai, Pham Cam Nam, Dinh Quy Huong
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4cp03537b Ngo Hong Cat Van, Nguyen Quang Trung, Pham Dinh Tu Tai, Pham Cam Nam, Dinh Quy Huong
|
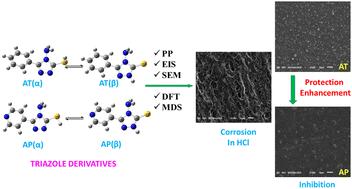
A comprehensive study was conducted, both experimentally and theoretically, to evaluate the corrosion inhibition ability of 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol (AT) and 4-amino-5-(pyridine-4-yl)-4H-1,2,4-triazole-3-thiol (AP) on mild steel. The findings show that AT and AP are potential mixed-type inhibitors in hydrochloric acid solution. At 293 K and a concentration of 5 × 10−3 M, AT and AP have efficiencies of 93.33% and 97.33%. When the temperature rises to 323 K, their efficiencies drop to 87.27% and 90.82%. The adsorption behavior of AT and AP on the steel surface conforms to the Langmuir adsorption isotherm. A key finding is the superior inhibition performance of AP over AT, attributed to its higher nitrogen heteroatom content, which enhances the interaction with the mild steel surface. Molecular dynamics simulations and quantum chemical calculations reveal that AP exhibits a notably higher binding energy (pAP-N20: 847.17 kJ mol−1) compared to AT (pAT-S18: 847.17 kJ mol−1). This study established a correlation between molecular structure, adsorption behavior, and corrosion inhibition efficiency, providing new insights into the design of effective corrosion inhibitors for industrial applications.
中文翻译:

评价三唑衍生物在酸性溶液中低碳钢缓蚀能力的实验和理论方法相结合
进行了一项全面的实验和理论研究,以评估 4-氨基-5-苯基-4 H-1,2,4-三唑-3-硫醇 (AT) 和 4-氨基-5-(吡啶-4-基)-4H-1,2,4-三唑-3-硫醇 (AP) 对低碳钢的腐蚀抑制能力。研究结果表明,AT 和 AP 是盐酸溶液中潜在的混合型抑制剂。在 293 K 和 5 × 10-3 M 浓度下,AT 和 AP 的效率分别为 93.33% 和 97.33%。当温度上升到 323 K 时,它们的效率下降到 87.27% 和 90.82%。AT 和 AP 在钢表面的吸附行为符合 Langmuir 吸附等温线。一个关键的发现是 AP 优于 AT 的抑制性能,这归因于其更高的氮杂原子含量,这增强了与低碳钢表面的相互作用。分子动力学模拟和量子化学计算表明,与 AT (pAT-S18: 847.17 kJ mol-1) 相比,AP 表现出明显更高的结合能 (pAP-N20: 847.17 kJ mol-1)。本研究建立了分子结构、吸附行为和缓蚀效率之间的相关性,为工业应用的有效缓蚀剂设计提供了新的见解。
更新日期:2024-11-07
中文翻译:

评价三唑衍生物在酸性溶液中低碳钢缓蚀能力的实验和理论方法相结合
进行了一项全面的实验和理论研究,以评估 4-氨基-5-苯基-4 H-1,2,4-三唑-3-硫醇 (AT) 和 4-氨基-5-(吡啶-4-基)-4H-1,2,4-三唑-3-硫醇 (AP) 对低碳钢的腐蚀抑制能力。研究结果表明,AT 和 AP 是盐酸溶液中潜在的混合型抑制剂。在 293 K 和 5 × 10-3 M 浓度下,AT 和 AP 的效率分别为 93.33% 和 97.33%。当温度上升到 323 K 时,它们的效率下降到 87.27% 和 90.82%。AT 和 AP 在钢表面的吸附行为符合 Langmuir 吸附等温线。一个关键的发现是 AP 优于 AT 的抑制性能,这归因于其更高的氮杂原子含量,这增强了与低碳钢表面的相互作用。分子动力学模拟和量子化学计算表明,与 AT (pAT-S18: 847.17 kJ mol-1) 相比,AP 表现出明显更高的结合能 (pAP-N20: 847.17 kJ mol-1)。本研究建立了分子结构、吸附行为和缓蚀效率之间的相关性,为工业应用的有效缓蚀剂设计提供了新的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号