当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and stereoselective azidation of activated N-allenamides: an entry to α, β, γ and δ-amido-azides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4qo01802h Dorian Schutz, Maxime Hourtoule, Laurence Miesch
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4qo01802h Dorian Schutz, Maxime Hourtoule, Laurence Miesch
|
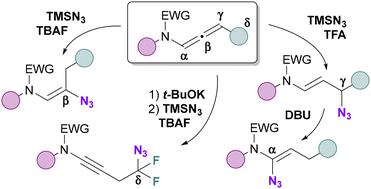
A totally controlled regiodivergent azidation of activated N-allenamides is presented. Using TMSN3/TBAF, β-azidation of N-allenamides occurs exclusively, yielding vinyl azides. Conversely, employing a TFA/TMSN3 mixture results solely in the formation of γ-azides. A subsequent [1,3] azide shift of the latter with DBU produces α-amido vinyl azides. Additionally, δ-difluorinated azides featuring an ynamide are selectively synthesized from ene-ynamides. The practical applicability of these transformations is demonstrated through the formation of cyanide derivatives, trifluoromethyl ketones and primary enamines.
中文翻译:

活化的 N-烯酰胺的区域选择性和立体选择性叠氮化:α、β、γ 和 δ-酰胺叠氮化物的入口
提出了活化的 N-烯酰胺的完全受控区域发散叠氮化。使用 TMSN3/TBAF,N-烯酰胺的 β 叠氮化反应仅发生,产生乙烯基叠氮化物。相反,使用 TFA/TMSN3 混合物仅导致 γ-叠氮化物的形成。随后 [1,3] 后者与 DBU 的叠氮化物偏移产生α-酰胺基叠氮化物。此外,具有烯酰胺的 δ-二氟叠氮化物是从烯-酰胺选择性合成的。这些转化的实际适用性通过氰化物衍生物、三氟甲基酮和伯烯胺的形成来证明。
更新日期:2024-11-06
中文翻译:

活化的 N-烯酰胺的区域选择性和立体选择性叠氮化:α、β、γ 和 δ-酰胺叠氮化物的入口
提出了活化的 N-烯酰胺的完全受控区域发散叠氮化。使用 TMSN3/TBAF,N-烯酰胺的 β 叠氮化反应仅发生,产生乙烯基叠氮化物。相反,使用 TFA/TMSN3 混合物仅导致 γ-叠氮化物的形成。随后 [1,3] 后者与 DBU 的叠氮化物偏移产生α-酰胺基叠氮化物。此外,具有烯酰胺的 δ-二氟叠氮化物是从烯-酰胺选择性合成的。这些转化的实际适用性通过氰化物衍生物、三氟甲基酮和伯烯胺的形成来证明。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号