Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constructing self-standing Fe2O3-Pt/NF nanoflowers with synergistic active sites for efficient electrocatalytic overall (sea) water splitting
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4nr03572k Weiping Xiao, Yue Zhang, Changwang Ke, Qin Zhao, Fengyan Han, Junpo Guo, Xiaofei Yang
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4nr03572k Weiping Xiao, Yue Zhang, Changwang Ke, Qin Zhao, Fengyan Han, Junpo Guo, Xiaofei Yang
|
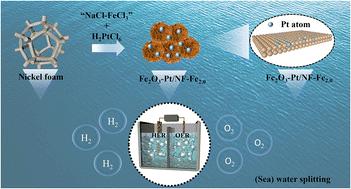
Designing cost-effective and highly stable heterostructures with synergistic active sites could simultaneously catalyze the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) for (sea) water splitting. However, there are still challenges in maintaining the catalytic performance of individual materials and in constructing intimate interfaces. Herein, a novel corrosion engineering method is provided to prepare self-standing Fe2O3-Pt/NF nanoflowers where ultra-small amounts of Pt combined with Fe2O3 are in situ grown on nickel foam (NF) in the corrosion system of “H2PtCl6–NaCl–FeCl3”. The synthesized Fe2O3-Pt/NF shows the presence of a Pt–O bond, which can regulate the electronic structure of the active sites and optimize the binding energy of the reaction intermediates, leading to an improvement in catalytic performance. Compared with Pt/NF and FeOOH/NF, the Fe2O3-Pt/NF heterostructure exhibits remarkable electrocatalytic activities with overpotentials reaching 94 mV and 265 mV for the HER and OER, respectively, at a high current density of 100 mA cm−2 in alkaline solution. Furthermore, the self-assembled electrolytic cell employing Fe2O3-Pt/NF as the bifunctional electrode only requires potentials of 1.60 V and 1.61 V to achieve a current density of 100 mA cm−2 in overall water and seawater splitting, respectively. This material remained stable for 10 hours without obvious attenuation, indicating its good environmental adaptability and stability. Specifically, the enhanced catalytic activity and stability can be ascribed to the abundant active sites of nanoflowers, fast electron transfer rate of intimate interfaces, and strong electronic interaction between Pt atoms and Fe2O3. This work provides a new insight into the construction of highly efficient co-catalysts with intimate interfaces based on corrosion engineering methods.
中文翻译:

构建具有协同活性位点的自立式 Fe2O3-Pt/NF 纳米花,用于高效电催化整体(海洋)分解水
设计具有协同活性位点的具有成本效益且高度稳定的异质结构可以同时催化析氧反应 (OER) 和析氢反应 (HER) 进行(海洋)水分解。然而,在保持单个材料的催化性能和构建亲密界面方面仍然存在挑战。本文提供了一种新型的腐蚀工程方法来制备自立式 Fe2O3-Pt/NF 纳米花,其中超少量的 Pt 与 Fe2O3 结合在“H2PtCl 6-NaCl-FeCl 3”腐蚀系统中的泡沫镍 (NF) 上原位生长。合成的 Fe2O3-Pt/NF 显示出 Pt-O 键的存在,它可以调节活性位点的电子结构并优化反应中间体的结合能,从而提高催化性能。与 Pt/NF 和 FeOOH/NF 相比,Fe2O3-Pt/NF 异质结构表现出显著的电催化活性,在碱性溶液中 100 mA cm-2 的高电流密度下,HER 和 OER 的过电位分别达到 94 mV 和 265 mV。此外,采用 Fe2O3-Pt/NF 作为双功能电极的自组装电解槽只需要 1.60 V 和 1.61 V 的电位,即可在整体水和海水分解中分别实现 100 mA cm-2 的电流密度。 该材料保持稳定 10 h 且无明显衰减,表明其具有良好的环境适应性和稳定性。具体来说,增强的催化活性和稳定性可归因于纳米花丰富的活性位点、亲密界面的快速电子转移速率以及 Pt 原子与 Fe2O3 之间的强电子相互作用。这项工作为基于腐蚀工程方法构建具有紧密界面的高效助催化剂提供了新的思路。
更新日期:2024-11-04
中文翻译:

构建具有协同活性位点的自立式 Fe2O3-Pt/NF 纳米花,用于高效电催化整体(海洋)分解水
设计具有协同活性位点的具有成本效益且高度稳定的异质结构可以同时催化析氧反应 (OER) 和析氢反应 (HER) 进行(海洋)水分解。然而,在保持单个材料的催化性能和构建亲密界面方面仍然存在挑战。本文提供了一种新型的腐蚀工程方法来制备自立式 Fe2O3-Pt/NF 纳米花,其中超少量的 Pt 与 Fe2O3 结合在“H2PtCl 6-NaCl-FeCl 3”腐蚀系统中的泡沫镍 (NF) 上原位生长。合成的 Fe2O3-Pt/NF 显示出 Pt-O 键的存在,它可以调节活性位点的电子结构并优化反应中间体的结合能,从而提高催化性能。与 Pt/NF 和 FeOOH/NF 相比,Fe2O3-Pt/NF 异质结构表现出显著的电催化活性,在碱性溶液中 100 mA cm-2 的高电流密度下,HER 和 OER 的过电位分别达到 94 mV 和 265 mV。此外,采用 Fe2O3-Pt/NF 作为双功能电极的自组装电解槽只需要 1.60 V 和 1.61 V 的电位,即可在整体水和海水分解中分别实现 100 mA cm-2 的电流密度。 该材料保持稳定 10 h 且无明显衰减,表明其具有良好的环境适应性和稳定性。具体来说,增强的催化活性和稳定性可归因于纳米花丰富的活性位点、亲密界面的快速电子转移速率以及 Pt 原子与 Fe2O3 之间的强电子相互作用。这项工作为基于腐蚀工程方法构建具有紧密界面的高效助催化剂提供了新的思路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号