European Journal of Nuclear Medicine and Molecular Imaging ( IF 8.6 ) Pub Date : 2024-11-04 , DOI: 10.1007/s00259-024-06959-5 Alexander Gäble, Alexander Dierks, Andreas Rinscheid, Marianne Patt, Georgine Wienand, Christian H. Pfob, Malte Kircher, Kazuhito Fukushima, Ana Antić Nikolić, Johanna S. Enke, Tilman Janzen, Julie Steinestel, Hildegard Kempter, Martin Trepel, Dorothea Weckermann, Constantin Lapa, Ralph A. Bundschuh
|
Purpose
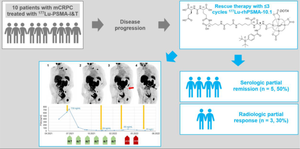
Radioligand therapy is an increasingly important option for the treatment of metastatic castrate-resistant prostate cancer (mCRPC). Radiohybrid ligands targeting prostate-specific membrane antigen (PSMA) are a novel group of theranostic radioligand therapy agents for which higher tumour absorbed radiation doses have been demonstrated compared to established PSMA ligands. Here, we report data from ten patients who were treated within a compassionate use program with the radiohybrid PSMA-ligand [177Lu]Lu-rhPSMA-10.1 after experiencing disease progression under treatment with [177Lu]Lu-PSMA-I&T.
Methods
Ten patients with advanced PSMA-positive prostate cancer who showed progression under treatment with [177Lu]Lu-PSMA-I&T received up to three cycles of rescue therapy with [177Lu]Lu-rhPSMA-10.1 (7.4–8.1 GBq per cycle). Efficacy (PSA response according to PCWG3 and RECIP) and overall survival were evaluated. Adverse events were recorded from first application.
Results
Despite progression with [177Lu]Lu-PSMA-I&T, after the first cycle of [177Lu]Lu-rhPSMA-10.1 rescue therapy, five patients (50%) showed a decrease in serum PSA level. In imaging, three of the ten patients (30%) showed a partial radiologic response. Four of the five patients with a decrease of serum PSA under [177Lu]Lu-rhPSMA-10.1 had initially responded to treatment with [177Lu]Lu-PSMA-I&T but had become resistant. However, the remaining patient had shown continuous disease progression during [177Lu]Lu-PSMA-I&T therapy but showed an immediate response to [177Lu]Lu-rhPSMA-10.1. The additional treatment with [177Lu]Lu-rhPSMA-10.1 was generally well tolerated by all patients.
Conclusions
Patients showing tumour progression while receiving [177Lu]Lu-PSMA-I&T radioligand therapy may benefit from rescue therapy with the novel radiohybrid PSMA ligand, [177Lu]Lu-rhPSMA-10.1. Higher tumour absorbed radiation doses with [177Lu]Lu-rhPSMA-10.1 may overcome primary and acquired radiation resistance.
Graphical Abstract
中文翻译:

对 [177Lu]Lu-PSMA-I&T 原发性或获得性耐药患者使用 [177Lu]Lu-rhPSMA-10.1 进行挽救治疗的经验
目的
放射配体治疗是治疗转移性去势抵抗性前列腺癌 (mCRPC) 的一种越来越重要的选择。靶向前列腺特异性膜抗原 (PSMA) 的放射性杂交配体是一组新型的治疗诊断放射配体治疗剂,与已建立的 PSMA 配体相比,其肿瘤吸收的辐射剂量更高。在这里,我们报告了 10 名患者的数据,这些患者在接受 [177Lu]Lu-PSMA-I&T 治疗后出现疾病进展,在同情使用计划中接受了放射性杂交 PSMA 配体 [177Lu]Lu-rhPSMA-10.1 的治疗。
方法
10 名晚期 PSMA 阳性前列腺癌患者在 [177Lu]Lu-PSMA-I&T 治疗下出现进展,接受了多达三个周期的 [177Lu]Lu-rhPSMA-10.1 挽救治疗(每个周期 7.4-8.1 GBq)。评估疗效 (根据 PCWG3 和 RECIP 的 PSA 反应) 和总生存期。从第一次申请开始记录不良事件。
结果
尽管 [177Lu]Lu-PSMA-I&T 进展,但在 [177Lu]Lu-rhPSMA-10.1 挽救治疗的第一个周期后,5 名患者 (50%) 显示血清 PSA 水平降低。在影像学检查中,10 例患者中有 3 例 (30%) 显示部分放射学反应。在 [177Lu]Lu-rhPSMA-10.1 下血清 PSA 降低的 5 例患者中,有 4 例最初对 [177Lu]Lu-PSMA-I&T 治疗有反应,但已变得耐药。然而,其余患者在 [177Lu]Lu-PSMA-I&T 治疗期间表现出持续的疾病进展,但对 [177Lu]Lu-rhPSMA-10.1 表现出即时反应。所有患者通常对 [177Lu]Lu-rhPSMA-10.1 的额外治疗耐受性良好。
结论
在接受 [177Lu]Lu-PSMA-I&T 放射配体治疗时出现肿瘤进展的患者可能受益于新型放射性杂交 PSMA 配体 [177Lu]Lu-rhPSMA-10.1 的挽救治疗。[177Lu]Lu-rhPSMA-10.1 的较高肿瘤吸收辐射剂量可以克服原发性和获得性辐射耐药性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号