当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed reductive 1,2-alkylarylation of alkenes via a 1,5-hydrogen atom transfer (HAT) cascade
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4qo01875c Xi Chen, Qiang Wang, Xiao-Ping Gong, Rui-Qiang Jiao, Xue-Yuan Liu, Yong-Min Liang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4qo01875c Xi Chen, Qiang Wang, Xiao-Ping Gong, Rui-Qiang Jiao, Xue-Yuan Liu, Yong-Min Liang
|
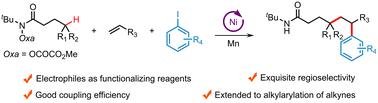
N-centered radical-mediated remote C(sp3)–H functionalization via HAT processes has been successfully applied in the difunctionalization of alkenes, serving as an elegant and robust method to convert readily available alkenes into various functionalized molecules. However, HAT strategy-enabled difunctionalization of alkenes using electrophiles as functionalizing reagents remains underexplored. In this study, we report a nickel-catalyzed regioselective reductive three-component 1,2-alkylarylation of alkenes with O-oxalate hydroxamic acid esters and aryl iodides. This radical addition/cross-coupling cascade reaction involves amidyl radical-triggered intramolecular 1,5-HAT and nickel-catalyzed reductive coupling processes under mild reaction conditions with good coupling efficiency. Additionally, this approach can be extended to the reductive 1,2-alkylarylation of alkynes, providing an efficient method for the synthesis of multi-substituted alkenes from easily accessible starting materials.
中文翻译:

烯烃通过 1,5-氢原子转移 (HAT) 级联反应进行镍催化的还原性 1,2-烷基化反应
通过 HAT 过程的 N 中心自由基介导的远程 C(sp3)-H 官能团化已成功应用于烯烃的二官能团化,作为一种将现成的烯烃转化为各种官能团化分子的优雅而稳健的方法。然而,使用亲电试剂作为官能化试剂的 HAT 策略实现烯烃的二官能化仍未得到充分探索。在这项研究中,我们报道了烯烃与邻草酸盐异羟肟酸酯和芳基碘化物的镍催化区域选择性还原三组分 1,2-烷基烷基化反应。这种自由基加成/交叉偶联反应涉及酰胺自由基触发的分子内 1,5-HAT 和镍催化的还原偶联过程,在温和的反应条件下具有良好的偶联效率。此外,这种方法可以扩展到炔烃的还原性 1,2-烷基烷基化,为从易于获得的起始材料合成多取代烯烃提供了一种有效的方法。
更新日期:2024-11-08
中文翻译:

烯烃通过 1,5-氢原子转移 (HAT) 级联反应进行镍催化的还原性 1,2-烷基化反应
通过 HAT 过程的 N 中心自由基介导的远程 C(sp3)-H 官能团化已成功应用于烯烃的二官能团化,作为一种将现成的烯烃转化为各种官能团化分子的优雅而稳健的方法。然而,使用亲电试剂作为官能化试剂的 HAT 策略实现烯烃的二官能化仍未得到充分探索。在这项研究中,我们报道了烯烃与邻草酸盐异羟肟酸酯和芳基碘化物的镍催化区域选择性还原三组分 1,2-烷基烷基化反应。这种自由基加成/交叉偶联反应涉及酰胺自由基触发的分子内 1,5-HAT 和镍催化的还原偶联过程,在温和的反应条件下具有良好的偶联效率。此外,这种方法可以扩展到炔烃的还原性 1,2-烷基烷基化,为从易于获得的起始材料合成多取代烯烃提供了一种有效的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号