当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
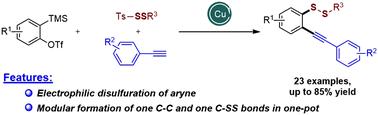
Copper-catalyzed alkynyldisulfuration of arynes: efficient access to unsymmetrical disulfides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4qo01844c Shuai Huang, Yumin Zhang, Yuekai Li, Chen-Ho Tung, Xin Li, Zhenghu Xu
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-05 , DOI: 10.1039/d4qo01844c Shuai Huang, Yumin Zhang, Yuekai Li, Chen-Ho Tung, Xin Li, Zhenghu Xu
|
This article investigates a copper-catalyzed alkynyl disulfuration reaction of arynes formed in situ that generates o-alkynyl aryl disulfides. The transformation proceeds through the in situ generation of arynes, followed by alkynylation under copper catalysis and disulfuration with a disulfide reagent, which results in the formation of one C–C bond and one C–SS bond in one pot under mild conditions. The reaction above can be readily employed to facilitate the introduction of a disulfide group and aryl alkynes into an aryne and thus provide a modular approach towards unsymmetrical disulfides.
中文翻译:

芳烃的铜催化炔基二硫化物:高效获得不对称的二硫化物
本文研究了原位形成的芳烃的铜催化炔基二硫化反应,该反应生成邻炔基芳基二硫化物。转化通过原位生成芳烃进行,然后在铜催化下进行炔基化反应,并用二硫化物试剂进行解硫,从而在温和条件下在一个罐中形成一个 C-C 键和一个 C-SS 键。上述反应可以很容易地用于促进将二硫键和芳基炔烃引入芳烷中,从而为不对称二硫键提供一种模块化方法。
更新日期:2024-11-05
中文翻译:

芳烃的铜催化炔基二硫化物:高效获得不对称的二硫化物
本文研究了原位形成的芳烃的铜催化炔基二硫化反应,该反应生成邻炔基芳基二硫化物。转化通过原位生成芳烃进行,然后在铜催化下进行炔基化反应,并用二硫化物试剂进行解硫,从而在温和条件下在一个罐中形成一个 C-C 键和一个 C-SS 键。上述反应可以很容易地用于促进将二硫键和芳基炔烃引入芳烷中,从而为不对称二硫键提供一种模块化方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号