当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystalline bilayer formation in homoleptic low-spin Fe(II) compounds with alkyl chain substituents
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4dt02667e Blaise L. Geoghegan, Spyridon Koutsoukos, Wasinee Phonsri, Keith S. Murray, Peter J. Cragg, Marcus K. Dymond, Ian A. Gass
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4dt02667e Blaise L. Geoghegan, Spyridon Koutsoukos, Wasinee Phonsri, Keith S. Murray, Peter J. Cragg, Marcus K. Dymond, Ian A. Gass
|
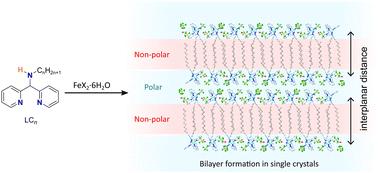
A series of asymmetric, homoleptic Fe(II) compounds based on the facially-binding tridentate ligand N-methyl-1,1-di(pyridin-2-yl)Cn-amine (LCn) (Cn = butyl, hexyl, octyl, decyl, dodecyl, tetradecyl and hexadecyl alkyl chains) with formula [FeII(LCn)2](X)2·solvate, where n = 4, 14 and X = BF4 (1C4 and 1C14) or n = 6, 8, 10, 12, 16 and X = CF3SO3 (1C6–1C12 and 1C16), are reported. Complexes 1C6 to 1C16 pack in crystalline bilayers in the solid state, forming hydrophobic and hydrophilic regions between adjacent layers of complexes. The combination of short Fe–N bond distances (∼2.00 Å) and SQUID magnetic susceptibility measurements show that the complexes are in the low-spin state across all measured temperature ranges. Differential scanning calorimetry confirmed phase transitions occur in compounds 1C6, 1C12, 1C14 and 1C16 upon heating from room temperature. The lack of any spin transitions and thermal stability conferred by thermogravimetric analysis over this temperature range suggest that these transitions are crystallographic in nature. 1H NMR studies show that the low-spin Fe(II) centres undergo partial conversion to paramagnetic species in solution. UV-vis spectroscopy in a range of common organic solvents show that the central Fe ions remain in the +2 oxidation state, suggesting that the increase in magnetic susceptibility in solution is likely due to partial spin-crossover, or due to speciation, in which a proportion of the compounds are high-spin Fe(II) complexes.
中文翻译:

具有烷基链取代基的同源低自旋 Fe(II) 化合物中的结晶双层形成
一系列基于表面结合三齿配体 N-甲基-1,1-二(吡啶-2-基)Cn-胺 (LCn) (Cn = 丁基、己基、辛基、癸基、十二烷基、十四烷基和十六烷基烷基链)的不对称同源 Fe(II) 化合物,分子式为 [FeII(LCn)2](X)2·溶剂化物,其中 n = 4、14 和 X = BF4(1C4 和 1C14)或 n = 6、8、10、12、16 和 X = CF3SO3(1C6–1C12 和 1C16),被报道。复合物 1C6 至 1C16 在固态的结晶双层中堆积,在复合物的相邻层之间形成疏水和亲水区域。短 Fe-N 键距离 (∼2.00 Å) 和 SQUID 磁化率测量相结合,表明复合物在所有测量温度范围内都处于低自旋状态。差示扫描量热法证实,化合物 1C6、1C12、1C14 和 1C16 在室温加热时发生相变。在此温度范围内,热重分析没有赋予任何自旋转变和热稳定性,这表明这些转变本质上是晶体学的。 1H NMR 研究表明,低自旋 Fe(II) 中心在溶液中部分转化为顺磁性物质。在一系列常见有机溶剂中的紫外-可见光谱显示,中心铁离子保持 +2 氧化态,这表明溶液中磁化率的增加可能是由于部分自旋交叉,或由于物种形成,其中一部分化合物是高自旋 Fe(II) 络合物。
更新日期:2024-11-05
中文翻译:

具有烷基链取代基的同源低自旋 Fe(II) 化合物中的结晶双层形成
一系列基于表面结合三齿配体 N-甲基-1,1-二(吡啶-2-基)Cn-胺 (LCn) (Cn = 丁基、己基、辛基、癸基、十二烷基、十四烷基和十六烷基烷基链)的不对称同源 Fe(II) 化合物,分子式为 [FeII(LCn)2](X)2·溶剂化物,其中 n = 4、14 和 X = BF4(1C4 和 1C14)或 n = 6、8、10、12、16 和 X = CF3SO3(1C6–1C12 和 1C16),被报道。复合物 1C6 至 1C16 在固态的结晶双层中堆积,在复合物的相邻层之间形成疏水和亲水区域。短 Fe-N 键距离 (∼2.00 Å) 和 SQUID 磁化率测量相结合,表明复合物在所有测量温度范围内都处于低自旋状态。差示扫描量热法证实,化合物 1C6、1C12、1C14 和 1C16 在室温加热时发生相变。在此温度范围内,热重分析没有赋予任何自旋转变和热稳定性,这表明这些转变本质上是晶体学的。 1H NMR 研究表明,低自旋 Fe(II) 中心在溶液中部分转化为顺磁性物质。在一系列常见有机溶剂中的紫外-可见光谱显示,中心铁离子保持 +2 氧化态,这表明溶液中磁化率的增加可能是由于部分自旋交叉,或由于物种形成,其中一部分化合物是高自旋 Fe(II) 络合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号