当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Beyond the triple bond: unlocking dinitrogen activation with tailored superbase phosphines
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4dt02703e Vilakkathala U. Krishnapriya, Cherumuttathu H. Suresh
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-04 , DOI: 10.1039/d4dt02703e Vilakkathala U. Krishnapriya, Cherumuttathu H. Suresh
|
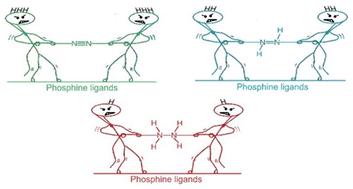
Activating atmospheric dinitrogen (N2), a molecule with a remarkably strong triple bond, remains a major challenge in chemistry. This theoretical study explores the potential of superbase phosphines, specifically those decorated with imidazolin-2-imine ((ImN)3P) and imidazolin-2-methylidene ((ImCH)3P) to facilitate N2 activation and subsequent hydrazine (H2NNH2) formation. Using density functional theory (DFT) at the M06L/6-311++G(d,p) level, we investigated the interactions between these phosphines and N2. Mono-phosphine–N2 complexes exhibit weak, noncovalent interactions (−0.6 to −7.1 kcal mol−1). Notably, two superbasic phosphines also form high-energy hypervalent complexes with N2, albeit at significantly higher energies. The superbasic nature and potential for the hypervalency of these phosphines lead to substantial N2 activation in bis-phosphine–N2 complexes, where N2 is “sandwiched” between two phosphine moieties through hypervalent P–N bonds. Among the phosphines studied, only (ImN)3P forms an exothermic sandwich complex with N2, stabilized by hydrogen bonding between the ImN substituents and the central N2 molecule. A two-step, exothermic hydrogen transfer pathway from (ImN)3P to N2 results in the formation of a bis-phosphine–diimine (HNNH) sandwich complex. Subsequent hydrogen transfer leads to the formation of a bis-phosphine–hydrazine (H2NNH2) complex, a process that, although endothermic, exhibits surmountable activation barriers. The relatively low energy requirements for this overall transformation suggest its potential feasibility under the optimized conditions. This theoretical exploration highlights the promise of superbase phosphines as a strategy for metal-free N2 activation, opening doors for the development of more efficient and sustainable nitrogen fixation and utilization methods.
中文翻译:

超越三键:使用定制的超碱基膦解锁二氮活化
激活大气中的二氮 (N2) 是一种具有非常强的三键的分子,仍然是化学中的一项重大挑战。这项理论研究探讨了超碱基膦的潜力,特别是那些用咪唑啉-2-亚胺 ((ImN)3P) 和咪唑啉-2-甲基亚胺 ((ImCH)3P) 修饰的膦,以促进 N2 活化和随后的肼 (H2NNH2) 形成。使用 M06L/6-311++G(d,p) 水平的密度泛函理论 (DFT),我们研究了这些膦与 N2 之间的相互作用。单膦-N2 配合物表现出弱的非共价相互作用 (-0.6 至 -7.1 kcal mol-1)。值得注意的是,两种超碱性膦也与 N2 形成高能高价络合物,尽管能量要高得多。这些膦的超碱性和高价性的可能性导致双膦-N2 复合物中的 N2 大量激活,其中 N2 通过高价 P-N 键“夹”在两个膦部分之间。在研究的膦中,只有 (ImN)3P 与 N2 形成放热三明治复合物,通过 ImN 取代基和中心 N2 分子之间的氢键稳定。从 (ImN)3P 到 N2 的两步放热氢转移途径导致形成双膦-二亚胺 (HNNH) 夹心复合物。 随后的氢转移导致双膦-肼 (H2NNH2) 络合物的形成,这一过程虽然是吸热的,但表现出可克服的活化障碍。这种整体改造的能源需求相对较低,这表明它在优化条件下具有潜在的可行性。这项理论探索强调了超碱膦作为无金属 N2 活化策略的前景,为开发更高效、更可持续的固氮和利用方法打开了大门。
更新日期:2024-11-04
中文翻译:

超越三键:使用定制的超碱基膦解锁二氮活化
激活大气中的二氮 (N2) 是一种具有非常强的三键的分子,仍然是化学中的一项重大挑战。这项理论研究探讨了超碱基膦的潜力,特别是那些用咪唑啉-2-亚胺 ((ImN)3P) 和咪唑啉-2-甲基亚胺 ((ImCH)3P) 修饰的膦,以促进 N2 活化和随后的肼 (H2NNH2) 形成。使用 M06L/6-311++G(d,p) 水平的密度泛函理论 (DFT),我们研究了这些膦与 N2 之间的相互作用。单膦-N2 配合物表现出弱的非共价相互作用 (-0.6 至 -7.1 kcal mol-1)。值得注意的是,两种超碱性膦也与 N2 形成高能高价络合物,尽管能量要高得多。这些膦的超碱性和高价性的可能性导致双膦-N2 复合物中的 N2 大量激活,其中 N2 通过高价 P-N 键“夹”在两个膦部分之间。在研究的膦中,只有 (ImN)3P 与 N2 形成放热三明治复合物,通过 ImN 取代基和中心 N2 分子之间的氢键稳定。从 (ImN)3P 到 N2 的两步放热氢转移途径导致形成双膦-二亚胺 (HNNH) 夹心复合物。 随后的氢转移导致双膦-肼 (H2NNH2) 络合物的形成,这一过程虽然是吸热的,但表现出可克服的活化障碍。这种整体改造的能源需求相对较低,这表明它在优化条件下具有潜在的可行性。这项理论探索强调了超碱膦作为无金属 N2 活化策略的前景,为开发更高效、更可持续的固氮和利用方法打开了大门。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号