当前位置:
X-MOL 学术
›
Coord. Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A critical review of coordination chemistry of pyrimidine and pyridazine compounds: Bonding, chelation and corrosion inhibition
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-11-02 , DOI: 10.1016/j.ccr.2024.216285 Taiwo W. Quadri, Ekemini D. Akpan, Saheed E. Elugoke, Omar Dagdag, Nnaemeka J. Nnaji, Chandrabhan Verma, Lukman O. Olasunkanmi, Akram AlFantazi, Valentine Chikaodili Anadebe, Rakesh Chandra Barik, Eno E. Ebenso
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-11-02 , DOI: 10.1016/j.ccr.2024.216285 Taiwo W. Quadri, Ekemini D. Akpan, Saheed E. Elugoke, Omar Dagdag, Nnaemeka J. Nnaji, Chandrabhan Verma, Lukman O. Olasunkanmi, Akram AlFantazi, Valentine Chikaodili Anadebe, Rakesh Chandra Barik, Eno E. Ebenso
|
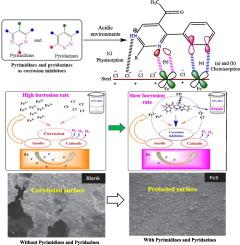
Metallic deterioration remains a formidable challenge in numerous industrial sectors, necessitating the continuous, intense search for effective, sustainable and non-toxic chemical inhibitors. Pyrimidines and pyridazines belong to a class of heterocycles that have garnered significant attention as potential corrosion inhibitors due to their versatile chemical configuration and promising protection performances. Notably, the nitrogen atoms in the six-membered heterocyclic ring of pyrimidine (C4 H4 N2 ), pyridazine (C4 H4 N2 ), and their derivatives are well known for their capacity to form coordination bonds with metal surfaces. Pyrimidine, pyridazine, and their derivatives form corrosion-inhibitive hydrophobic layers through their adsorption on the metal surfaces. The widespread conjugation of π-electrons enhances the durability and efficacy of the hydrophobic film. They demonstrate excellent inhibition efficiencies ranging from 70 to 100 % at low concentrations (<1 mM) for different metal/electrolyte systems. This review provides an overview of the properties and application of these heterocyclic compounds in chelation and coordination. Furthermore, their potential applications as aqueous phase inhibitors for different metal/electrolyte systems were comprehensively covered. Using experimental and computational tools, emphasis was placed on the coordination chemistry of pyrimidine and pyridazine, and its adsorption behaviour against metallic degradation in diverse corrosive environments was highlighted. Finally, patent literature on the effectiveness of pyrimidine and pyridazine and future perspectives were presented.
中文翻译:

嘧啶和哒嗪化合物配位化学的批判性综述:键合、螯合和腐蚀抑制
金属劣化仍然是许多工业部门面临的巨大挑战,需要持续、密集地寻找有效、可持续和无毒的化学抑制剂。嘧啶和嘧啶属于一类杂环,由于其多功能的化学构型和有前途的保护性能,它们作为潜在的缓蚀剂而受到广泛关注。值得注意的是,嘧啶 (C4H4N2)、哒嗪 (C4H4N2) 及其衍生物的六元杂环中的氮原子以其与金属表面形成配位键的能力而闻名。嘧啶、哒嗪及其衍生物通过吸附在金属表面形成腐蚀抑制疏水层。π电子的广泛共轭增强了疏水膜的耐用性和功效。对于不同的金属/电解质系统,它们在低浓度 (<1 mM) 下表现出优异的抑制效率,范围为 70% 至 100%。本文概述了这些杂环化合物在螯合和配位中的特性和应用。此外,还全面介绍了它们作为不同金属/电解质体系的水相抑制剂的潜在应用。利用实验和计算工具,重点研究了嘧啶和哒嗪的配位化学,强调了其在各种腐蚀环境中对金属降解的吸附行为。最后,介绍了关于嘧啶和哒嗪的有效性和未来前景的专利文献。
更新日期:2024-11-02
中文翻译:

嘧啶和哒嗪化合物配位化学的批判性综述:键合、螯合和腐蚀抑制
金属劣化仍然是许多工业部门面临的巨大挑战,需要持续、密集地寻找有效、可持续和无毒的化学抑制剂。嘧啶和嘧啶属于一类杂环,由于其多功能的化学构型和有前途的保护性能,它们作为潜在的缓蚀剂而受到广泛关注。值得注意的是,嘧啶 (C4H4N2)、哒嗪 (C4H4N2) 及其衍生物的六元杂环中的氮原子以其与金属表面形成配位键的能力而闻名。嘧啶、哒嗪及其衍生物通过吸附在金属表面形成腐蚀抑制疏水层。π电子的广泛共轭增强了疏水膜的耐用性和功效。对于不同的金属/电解质系统,它们在低浓度 (<1 mM) 下表现出优异的抑制效率,范围为 70% 至 100%。本文概述了这些杂环化合物在螯合和配位中的特性和应用。此外,还全面介绍了它们作为不同金属/电解质体系的水相抑制剂的潜在应用。利用实验和计算工具,重点研究了嘧啶和哒嗪的配位化学,强调了其在各种腐蚀环境中对金属降解的吸附行为。最后,介绍了关于嘧啶和哒嗪的有效性和未来前景的专利文献。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号