当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of hybrid aptamers-engineered PROTACs for degrading VEGF165 in both tumor- and vascular endothelial cells
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-02 , DOI: 10.1016/j.ejmech.2024.117027 Ziting Feng, Duoli Xie, Fang Qiu, Jie Huang, Zhuqian Wang, Chao Liang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-02 , DOI: 10.1016/j.ejmech.2024.117027 Ziting Feng, Duoli Xie, Fang Qiu, Jie Huang, Zhuqian Wang, Chao Liang
|
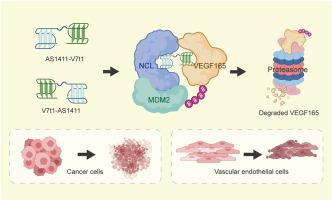
Tumors and angiogenesis are connected through a complex interplay. VEGF165, generated from both tumor and vascular endothelial cells, serves as a mutual benefit for both cell types. Therapeutic approaches modulating VEGF165 have been proposed as promising antitumor therapies. PROTACs are bifunctional molecules that exploit the intracellular ubiquitin-proteasome system to degrade specific proteins. To date, there are no targeted PROTACs designed to degrade VEGF165 in both tumor and vascular endothelial cells. The aptamer AS1411 is notable for its ability to selectively recognize and enter both tumor and vascular endothelial cells by targeting the cell surface nucleolin (NCL). Moreover, AS1411 has also been repurposed as an intracellular recruiter of E3 ligase MDM2 via leveraging NCL as a molecular bridge. In this study, we conjugated AS1411 with a VEGF165-specific aptamer V7t1, creating hybrid aptamers-engineered PROTACs. The PROTACs demonstrate remarkable selectivity for both tumor and vascular endothelial cells and facilitate the ubiquitination and proteasomal degradation of VEGF165. The PROTACs inhibit the growth of tumor cells and also impede angiogenesis, without causing toxicity to normal tissues. The hybrid aptamers-engineered PROTACs provide an avenue for disrupting the tumor-angiogenesis interplay through modulation of VEGF165 in both tumor and vascular endothelial cells.
中文翻译:

开发杂交适体工程 PROTAC 以降解肿瘤和血管内皮细胞中的VEGF165
肿瘤和血管生成通过复杂的相互作用联系在一起。VEGF165 由肿瘤和血管内皮细胞产生,对两种细胞类型都有利。调节 VEGF165 的治疗方法已被提议为有前途的抗肿瘤疗法。PROTAC 是双功能分子,利用细胞内泛素-蛋白酶体系统降解特定蛋白质。迄今为止,还没有旨在降解肿瘤和血管内皮细胞中 VEGF165 的靶向 PROTAC。适配体 AS1411 因其通过靶向细胞表面核仁素 (NCL) 选择性地识别和进入肿瘤和血管内皮细胞的能力而著称。此外,AS1411 还通过利用 NCL 作为分子桥,被重新用作 E3 连接酶 MDM2 的细胞内募集因子。在这项研究中,我们将 AS1411 与 VEGF165 特异性适配体 V7t1 偶联,产生杂交适体工程 PROTAC。PROTAC 对肿瘤和血管内皮细胞均表现出显著的选择性,并促进 VEGF165 的泛素化和蛋白酶体降解。PROTACs 抑制肿瘤细胞的生长,也阻碍血管生成,而不会对正常组织造成毒性。杂交适体工程 PROTAC 提供了一种途径,通过调节肿瘤和血管内皮细胞中的 VEGF165 来破坏肿瘤-血管生成相互作用。
更新日期:2024-11-02
中文翻译:

开发杂交适体工程 PROTAC 以降解肿瘤和血管内皮细胞中的VEGF165
肿瘤和血管生成通过复杂的相互作用联系在一起。VEGF165 由肿瘤和血管内皮细胞产生,对两种细胞类型都有利。调节 VEGF165 的治疗方法已被提议为有前途的抗肿瘤疗法。PROTAC 是双功能分子,利用细胞内泛素-蛋白酶体系统降解特定蛋白质。迄今为止,还没有旨在降解肿瘤和血管内皮细胞中 VEGF165 的靶向 PROTAC。适配体 AS1411 因其通过靶向细胞表面核仁素 (NCL) 选择性地识别和进入肿瘤和血管内皮细胞的能力而著称。此外,AS1411 还通过利用 NCL 作为分子桥,被重新用作 E3 连接酶 MDM2 的细胞内募集因子。在这项研究中,我们将 AS1411 与 VEGF165 特异性适配体 V7t1 偶联,产生杂交适体工程 PROTAC。PROTAC 对肿瘤和血管内皮细胞均表现出显著的选择性,并促进 VEGF165 的泛素化和蛋白酶体降解。PROTACs 抑制肿瘤细胞的生长,也阻碍血管生成,而不会对正常组织造成毒性。杂交适体工程 PROTAC 提供了一种途径,通过调节肿瘤和血管内皮细胞中的 VEGF165 来破坏肿瘤-血管生成相互作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号