当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-Methyleneisoindolin-1-one Assembly via Base- and CuI/l-Proline-Catalyzed Domino Reaction: Mechanism of Regioselective Anionic Cyclization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-11-03 00:00:00 , DOI: 10.1021/acs.joc.6b01904 Li Li 1 , Benjamin G. Janesko 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-11-03 00:00:00 , DOI: 10.1021/acs.joc.6b01904 Li Li 1 , Benjamin G. Janesko 2
Affiliation
|
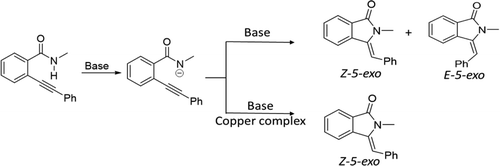
Anionic cyclization of o-alkynylbenzamides is proposed as a crucial step in many heterocycle syntheses. The cyclization can produce three products: Z-3-methylenisoindolin-1-one (Z-5-exo), E-3-methylenisoindolin-1-one (E-5-exo), and isoquinolinone (6-endo). Under base catalysis, the selectivity is generally poor. However, a copper-involved domino reaction of coupling and cyclization gives surprising selectivity for the thermodynamically disfavored Z-5-exo product (Org. Lett.2009, 11, 1309–1312). We study the selectivity of anionic cyclization in the presence of K2CO3 and copper-l-proline, using surveys of the experimental literature and density functional theory (DFT) calculations. The o-alkynylbenzamide is predicted to be readily deprotonated by many bases, with subsequent cyclization via nucleophilic attack of the amide N– to alkynyl. In the absence of copper, endo-exo selectivity is predicted to arise from substituent effects, while Z/E selectivity is a sensitive function of the tautomerization rate of an alkenyl anion intermediate. Most importantly, we predict that the remarkable selectivity of the copper-involved reaction occurs because copper-l-proline “locks” the alkene anion intermediates into the initially formed Z-5-exo configuration. Calculations on other metals suggest that soft Lewis acid additives provide a potential route to improved regiocontrol of other anionic cyclizations.
中文翻译:

3-亚甲基异吲哚啉-1-酮通过碱和CuI / l-脯氨酸催化的多米诺反应的组装:区域选择性阴离子环化的机理
提议将邻炔基苯甲酰胺的阴离子环化作为许多杂环合成中的关键步骤。环化可产生三种产物:Z -3-甲基异吲哚啉-1-酮(Z -5- exo),E -3-甲基异吲哚啉-1-酮(E -5- exo)和异喹啉酮(6- endo)。在碱催化下,选择性通常较差。然而,耦合和环化的铜参与多米诺反应得到令人惊讶的选择性为热力学上不受欢迎Ž -5 -外产物(有机化学快报。2009,11,1309年至1312年)。我们研究了在K 2 CO 3存在下阴离子环化的选择性和铜-1-脯氨酸,使用对实验文献的调查和密度泛函理论(DFT)的计算。预计邻炔基苯甲酰胺很容易被许多碱基去质子化,随后通过酰胺N –对炔基的亲核攻击而环化。在不存在铜的情况下,预计内-外选择性是由取代基效应引起的,而Z / E选择性是烯基阴离子中间体互变异构速率的敏感函数。最重要的是,我们预测发生涉及铜的反应的显着选择性是因为铜-1-脯氨酸将烯烃阴离子中间体“锁定”在最初形成的Z中-5-exo配置。对其他金属的计算表明,柔软的路易斯酸添加剂为改善其他阴离子环化的区域控制提供了一条潜在途径。
更新日期:2016-11-03
中文翻译:

3-亚甲基异吲哚啉-1-酮通过碱和CuI / l-脯氨酸催化的多米诺反应的组装:区域选择性阴离子环化的机理
提议将邻炔基苯甲酰胺的阴离子环化作为许多杂环合成中的关键步骤。环化可产生三种产物:Z -3-甲基异吲哚啉-1-酮(Z -5- exo),E -3-甲基异吲哚啉-1-酮(E -5- exo)和异喹啉酮(6- endo)。在碱催化下,选择性通常较差。然而,耦合和环化的铜参与多米诺反应得到令人惊讶的选择性为热力学上不受欢迎Ž -5 -外产物(有机化学快报。2009,11,1309年至1312年)。我们研究了在K 2 CO 3存在下阴离子环化的选择性和铜-1-脯氨酸,使用对实验文献的调查和密度泛函理论(DFT)的计算。预计邻炔基苯甲酰胺很容易被许多碱基去质子化,随后通过酰胺N –对炔基的亲核攻击而环化。在不存在铜的情况下,预计内-外选择性是由取代基效应引起的,而Z / E选择性是烯基阴离子中间体互变异构速率的敏感函数。最重要的是,我们预测发生涉及铜的反应的显着选择性是因为铜-1-脯氨酸将烯烃阴离子中间体“锁定”在最初形成的Z中-5-exo配置。对其他金属的计算表明,柔软的路易斯酸添加剂为改善其他阴离子环化的区域控制提供了一条潜在途径。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号