当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and evaluation of 4-(4-methyl-4H-1,2,4-triazol-3-yl)piperidine derivatives as potential glutaminyl cyclase isoenzyme inhibitors for the treatment of cancer
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-01 , DOI: 10.1016/j.ejmech.2024.117019 Qingqing Zhou, Zhenxin Wu, Feixia Qin, Pan He, Zhuoran Wang, Fangyi Zhu, Ying Gao, Wei Xiong, Chenyang Li, Haiqiang Wu
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-01 , DOI: 10.1016/j.ejmech.2024.117019 Qingqing Zhou, Zhenxin Wu, Feixia Qin, Pan He, Zhuoran Wang, Fangyi Zhu, Ying Gao, Wei Xiong, Chenyang Li, Haiqiang Wu
|
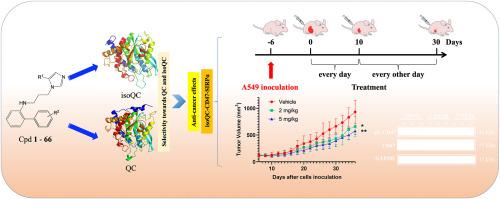
Upregulated glutaminyl cyclase isoenzyme (isoQC) contributes to cancer development by catalyzing pE-CD47 generation and thus enhancing CD47-SIRPα binding and subsequent “don't eat me” signals. We thus consider that isoQC could represent a novel target for cancer therapy. We previously prepared a series of diphenyl conjugated imidazole derivatives (DPCIs) and evaluated their use as glutaminyl cyclase (QC) inhibitors. Here, a new series of DPCIs was rationally designed and synthesized. As anticipated, the analogues exhibited considerably improved inhibitory potency against both QC and isoQC. Crucially, these chemicals exhibited marked selectivity toward isoQC. Further assessments established that one selected compound (27 ) did not affect the viability of A549, H1299, PC9, or HEK293T cells or the body weight of mice. This compound did, however, reduce pE-CD47 levels in infected A549 cells (isoQC_OE and isoQC_KD) and exhibited apparent anti-cancer effects in vivo by downregulating the level of pE-CD47 via the inhibition of isoQC activity. Taken together, these findings indicated that the compounds synthesized in this study could represent potential QC/isoQC inhibitors for the treatment of cancers.
中文翻译:

4-(4-甲基-4H-1,2,4-三唑-3-基)哌啶衍生物作为治疗癌症的潜在谷氨酰胺环化酶同工酶抑制剂的设计、合成和评价
上调的谷氨酰胺酰环化酶同工酶 (isoQC) 通过催化 pE-CD47 的产生,从而增强 CD47-SIRPα 结合和随后的“不要吃我”信号,从而促进癌症的发展。因此,我们认为 isoQC 可能代表癌症治疗的新靶点。我们之前制备了一系列二苯基共轭咪唑衍生物 (DPCI) 并评估了它们作为谷氨酰胺环化酶 (QC) 抑制剂的用途。在这里,合理地设计和综合了一系列新的 DPCI。正如预期的那样,类似物对 QC 和 isoQC 的抑制效力均表现出显著提高。至关重要的是,这些化学品对 isoQC 表现出显著的选择性。进一步评估确定,一种选定的化合物 (27) 不会影响 A549、H1299、PC9 或 HEK293T 细胞的活力或小鼠的体重。然而,该化合物确实降低了感染的 A549 细胞 (isoQC_OE 和 isoQC_KD) 中的 pE-CD47 水平,并通过抑制 isoQC 活性下调 pE-CD47 的水平,在体内表现出明显的抗癌作用。综上所述,这些发现表明本研究中合成的化合物可能代表治疗癌症的潜在 QC/isoQC 抑制剂。
更新日期:2024-11-01
中文翻译:

4-(4-甲基-4H-1,2,4-三唑-3-基)哌啶衍生物作为治疗癌症的潜在谷氨酰胺环化酶同工酶抑制剂的设计、合成和评价
上调的谷氨酰胺酰环化酶同工酶 (isoQC) 通过催化 pE-CD47 的产生,从而增强 CD47-SIRPα 结合和随后的“不要吃我”信号,从而促进癌症的发展。因此,我们认为 isoQC 可能代表癌症治疗的新靶点。我们之前制备了一系列二苯基共轭咪唑衍生物 (DPCI) 并评估了它们作为谷氨酰胺环化酶 (QC) 抑制剂的用途。在这里,合理地设计和综合了一系列新的 DPCI。正如预期的那样,类似物对 QC 和 isoQC 的抑制效力均表现出显著提高。至关重要的是,这些化学品对 isoQC 表现出显著的选择性。进一步评估确定,一种选定的化合物 (27) 不会影响 A549、H1299、PC9 或 HEK293T 细胞的活力或小鼠的体重。然而,该化合物确实降低了感染的 A549 细胞 (isoQC_OE 和 isoQC_KD) 中的 pE-CD47 水平,并通过抑制 isoQC 活性下调 pE-CD47 的水平,在体内表现出明显的抗癌作用。综上所述,这些发现表明本研究中合成的化合物可能代表治疗癌症的潜在 QC/isoQC 抑制剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号