当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light responsive defluorination-acyl fluoride exchange for photoclick labeling based on phenoxazine chromophores
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-01 , DOI: 10.1039/d4qo01870b Lijun Deng, Sitong Li, Cefei Zhang, Yuqiao Zhou, Zhishan Su, Changwei Hu, Xiaohu Zhao, Zhipeng Yu
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-01 , DOI: 10.1039/d4qo01870b Lijun Deng, Sitong Li, Cefei Zhang, Yuqiao Zhou, Zhishan Su, Changwei Hu, Xiaohu Zhao, Zhipeng Yu
|
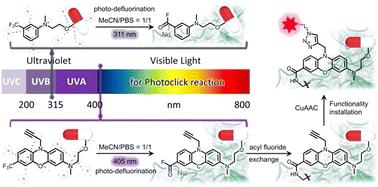
Photoclick chemistry represents an integration of photo- and click chemistry, enabling spatiotemporal control, high selectivity, and efficient conjugation. Photo-induced defluorination acyl fluoride exchange (photo-DAFEx), as a novel photoclick reaction, has emerged as a promising tool for the flourishing field of photoaffinity labeling (PAL) for drug discovery and the exploration of protein interactions. Currently, the first-generation photo-DAFEx reaction relies on the photo-defluorination of a monocyclic m-trifluoromethylaniline, and consequently the excitation wavelength (λex.) falls within the UV-B band (311 nm), limiting its widespread applications. Therefore, there is a high demand for the discovery of innovative cores that can expedite visible-light-induced photo-DAFEx reactions and for the exploration of their crosslinking capabilities. Herein, we report that the combination of phenoxazine chromophores with dialkylated amine auxochromes expands the excitation wavelength (λex.) of the photo-DAFEx reacion into the visible region (405 nm), enabling the multifunctional design of photoaffinity probes for in situ identification of drug–target interactions. By employing the phenoxazine-based photo-DAFEx reagent, we successfully developed potent PAL probes targeting hCA-II and BRD4, which can be activated with controllability using a 405 nm LED, thereby underscoring the potential of photo-DAFEx in advancing our understanding of protein–ligand interactions.
中文翻译:

基于吩噁嗪发色团的可见光响应脱氟-酰氟交换光click labeling
Photoclick 化学代表了 photo-click 化学和click化学的集成,可实现时空控制、高选择性和高效偶联。光诱导脱氟酰氟交换 (photo-DAFEx) 作为一种新型的光点击反应,已成为用于药物发现和蛋白质相互作用探索的光亲和标记 (PAL) 蓬勃发展领域的一种有前途的工具。目前,第一代光-DAFEx 反应依赖于单环间三氟甲基苯胺的光脱氟反应,因此激发波长 (λex.) 落在 UV-B 波段 (311 nm) 内,限制了其广泛应用。因此,对于发现可以加速可见光诱导的光 DAFEx 反应的创新核心以及探索它们的交联能力的需求很高。在此,我们报道了吩噁嗪发色团与二烷基化胺辅助色素的组合将光-DAFEx 反应的激发波长 (λex.) 扩展到可见区域 (405 nm),从而能够实现光亲和探针的多功能设计,用于原位鉴定药物-靶标相互作用。通过使用基于吩噁嗪的光 DAFEx 试剂,我们成功开发了靶向 hCA-II 和 BRD4 的强效 PAL 探针,可以使用 405 nm LED 可控激活,从而强调了光 DAFEx 在推进我们对蛋白质-配体相互作用的理解方面的潜力。
更新日期:2024-11-01
中文翻译:

基于吩噁嗪发色团的可见光响应脱氟-酰氟交换光click labeling
Photoclick 化学代表了 photo-click 化学和click化学的集成,可实现时空控制、高选择性和高效偶联。光诱导脱氟酰氟交换 (photo-DAFEx) 作为一种新型的光点击反应,已成为用于药物发现和蛋白质相互作用探索的光亲和标记 (PAL) 蓬勃发展领域的一种有前途的工具。目前,第一代光-DAFEx 反应依赖于单环间三氟甲基苯胺的光脱氟反应,因此激发波长 (λex.) 落在 UV-B 波段 (311 nm) 内,限制了其广泛应用。因此,对于发现可以加速可见光诱导的光 DAFEx 反应的创新核心以及探索它们的交联能力的需求很高。在此,我们报道了吩噁嗪发色团与二烷基化胺辅助色素的组合将光-DAFEx 反应的激发波长 (λex.) 扩展到可见区域 (405 nm),从而能够实现光亲和探针的多功能设计,用于原位鉴定药物-靶标相互作用。通过使用基于吩噁嗪的光 DAFEx 试剂,我们成功开发了靶向 hCA-II 和 BRD4 的强效 PAL 探针,可以使用 405 nm LED 可控激活,从而强调了光 DAFEx 在推进我们对蛋白质-配体相互作用的理解方面的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号