Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and functional studies of the EGF20-27 region reveal new features of the human Notch receptor important for optimal activation
Structure ( IF 4.4 ) Pub Date : 2024-11-01 , DOI: 10.1016/j.str.2024.10.012 Zhihan Bo, Thomas Rowntree, Steven Johnson, Hilman Nurmahdi, Richard J. Suckling, Johan Hill, Boguslawa Korona, Philip C. Weisshuhn, Devon Sheppard, Yao Meng, Shaoyan Liang, Edward D. Lowe, Susan M. Lea, Christina Redfield, Penny A. Handford
Structure ( IF 4.4 ) Pub Date : 2024-11-01 , DOI: 10.1016/j.str.2024.10.012 Zhihan Bo, Thomas Rowntree, Steven Johnson, Hilman Nurmahdi, Richard J. Suckling, Johan Hill, Boguslawa Korona, Philip C. Weisshuhn, Devon Sheppard, Yao Meng, Shaoyan Liang, Edward D. Lowe, Susan M. Lea, Christina Redfield, Penny A. Handford
|
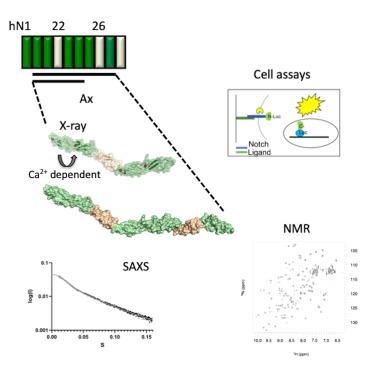
The Notch receptor is activated by the Delta/Serrate/Lag-2 (DSL) family of ligands. The organization of the extracellular signaling complex is unknown, although structures of Notch/ligand complexes comprising the ligand-binding region (LBR), and negative regulatory region (NRR) region, have been solved. Here, we investigate the human Notch-1 epidermal growth factor-like (EGF) 20-27 region, located between the LBR and NRR, and incorporating the Abruptex (Ax) region, associated with distinctive Drosophila phenotypes. Our analyses, using crystallography, NMR and small angle X-ray scattering (SAXS), support a rigid, elongated organization for EGF20-27 with the EGF20-21 linkage showing Ca2+ -dependent flexibility. In functional assays, Notch-1 variants containing Ax substitutions result in reduced ligand-dependent trans -activation. When cis -JAG1 was expressed, Notch activity differences between WT and Ca2+ -binding Ax variants were less marked than seen in the trans -activation assays alone, consistent with disruption of cis -inhibition. These data indicate the importance of Ca2+ -stabilized structure and suggest the balance of cis - and trans -interactions explains the effects of Drosophila Ax mutations.
中文翻译:

EGF20-27 区域的结构和功能研究揭示了人类 Notch 受体的新特征,对最佳激活很重要
Notch 受体被 Delta/Serrate/Lag-2 (DSL) 配体家族激活。细胞外信号复合物的组织尚不清楚,尽管已经解决了包含配体结合区 (LBR) 和负调节区 (NRR) 区域的 Notch/配体复合物的结构。在这里,我们研究了人类 Notch-1 表皮生长因子样 (EGF) 20-27 区域,位于 LBR 和 NRR 之间,并结合了与独特的果蝇表型相关的 Abruptex (Ax) 区域。我们使用晶体学、NMR 和小角 X 射线散射 (SAXS) 进行分析,支持 EGF20-27 的刚性、细长组织,EGF20-21 键显示出 Ca2+ 依赖性柔韧性。在功能测定中,含有 Ax 取代的 Notch-1 变体导致配体依赖性反式激活减少。当 cis-JAG1 表达时,WT 和 Ca2+ 结合 Ax 变体之间的 Notch 活性差异不如单独反式激活试验中观察到的明显,这与顺式抑制的破坏一致。这些数据表明了 Ca2+ 稳定结构的重要性,并表明顺式和反式相互作用的平衡解释了果蝇 Ax 突变的影响。
更新日期:2024-11-01
中文翻译:

EGF20-27 区域的结构和功能研究揭示了人类 Notch 受体的新特征,对最佳激活很重要
Notch 受体被 Delta/Serrate/Lag-2 (DSL) 配体家族激活。细胞外信号复合物的组织尚不清楚,尽管已经解决了包含配体结合区 (LBR) 和负调节区 (NRR) 区域的 Notch/配体复合物的结构。在这里,我们研究了人类 Notch-1 表皮生长因子样 (EGF) 20-27 区域,位于 LBR 和 NRR 之间,并结合了与独特的果蝇表型相关的 Abruptex (Ax) 区域。我们使用晶体学、NMR 和小角 X 射线散射 (SAXS) 进行分析,支持 EGF20-27 的刚性、细长组织,EGF20-21 键显示出 Ca2+ 依赖性柔韧性。在功能测定中,含有 Ax 取代的 Notch-1 变体导致配体依赖性反式激活减少。当 cis-JAG1 表达时,WT 和 Ca2+ 结合 Ax 变体之间的 Notch 活性差异不如单独反式激活试验中观察到的明显,这与顺式抑制的破坏一致。这些数据表明了 Ca2+ 稳定结构的重要性,并表明顺式和反式相互作用的平衡解释了果蝇 Ax 突变的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号