Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zr-NMOF tagged with heterobifunctionalized aptamers for highly sensitive, multiplexed and rapid imaging mass cytometry
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-01 , DOI: 10.1039/d4nr03477e Kaiwen Bao, Xiaoxiang Chen, Rui Chen, Yingying Gao, Jingqi Dang, Jie He, Ziqing Yuan, Yiyang Li, Adeleh Divsalar, Edwin Cheung, Guangxia Shen, Xianting Ding
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-01 , DOI: 10.1039/d4nr03477e Kaiwen Bao, Xiaoxiang Chen, Rui Chen, Yingying Gao, Jingqi Dang, Jie He, Ziqing Yuan, Yiyang Li, Adeleh Divsalar, Edwin Cheung, Guangxia Shen, Xianting Ding
|
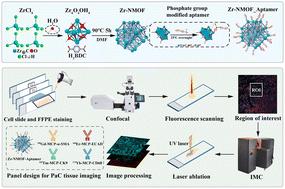
Imaging mass cytometry (IMC) permits high-dimensional single-cell spatial proteomics by harnessing mass tags to replace conventional fluorescence tags. However, the current IMC technique commonly adopts metal-chelated polymer (MCP) tags, which are limited in sensitivity, multiplicity and data acquisition speed. Here, we demonstrate nanometal–organic framework (NMOF) tags, which could concurrently augment IMC's sensitivity, multiplicity, and acquisition speed. We designed and synthesized uniform-sized Zr-NMOFs (∼31 nm, PDI < 0.1) and then functionalized them with heterobifunctionalized aptamers containing phosphate groups and fluorescent moieties to generate Zr-NMOF_Aptamer probes. Such functionalization enabled direct ligand exchange with zirconium ions on Zr-NMOFs, thus allowing for concurrent fluorescence and mass signal acquisitions. The fluorescence signal enabled large-scale rapid imaging to quickly locate the region-of-interest, therefore significantly reducing IMC's blind scanning time and compensating for IMC's lower resolution. Meanwhile, the Zr-NMOF_Aptamer probe exhibited specific molecular recognition and a fourfold enhancement in signal amplification over the commercial MCP probe. Additionally, we showed that Zr-NMOF_Aptamer probes were compatible with commercial MCP probes for high-multiplex co-staining in IMC analysis. The Zr-NMOF_Aptamer probe represents a promising development of next-generation molecular probes for spatial proteomics with IMC.
中文翻译:

用异双功能化适配子标记的 Zr-NMOF,用于高灵敏度、多重和快速成像质谱流式细胞术
质谱流式细胞术 (IMC) 通过利用质谱标签取代传统荧光标签,实现高维单细胞空间蛋白质组学。然而,目前的 IMC 技术通常采用金属螯合聚合物 (MCP) 标签,这些标签在灵敏度、多重性和数据采集速度方面受到限制。在这里,我们展示了纳米金属-有机框架 (NMOF) 标签,它可以同时提高 IMC 的灵敏度、多样性和采集速度。我们设计并合成了大小均匀的 Zr-NMOFs (∼31 nm,PDI < 0.1),然后用含有磷酸基团和荧光部分的异双功能化适体对其进行功能化,以生成 Zr-NMOF_Aptamer 探针。这种官能化使得与 Zr-NMOF 上的锆离子直接配体交换成为可能,从而可以同时进行荧光和质谱信号采集。荧光信号使大规模快速成像能够快速定位感兴趣区域,从而显著减少 IMC 的盲扫描时间并补偿 IMC 的较低分辨率。同时,Zr-NMOF_Aptamer 探针表现出特异性分子识别,信号放大能力比市售 MCP 探针高出四倍。此外,我们还表明 Zr-NMOF_Aptamer 探针与 IMC 分析中用于高多重共染色的市售 MCP 探针兼容。Zr-NMOF_Aptamer 探针代表了 IMC 空间蛋白质组学下一代分子探针的有前途的发展。
更新日期:2024-11-01
中文翻译:

用异双功能化适配子标记的 Zr-NMOF,用于高灵敏度、多重和快速成像质谱流式细胞术
质谱流式细胞术 (IMC) 通过利用质谱标签取代传统荧光标签,实现高维单细胞空间蛋白质组学。然而,目前的 IMC 技术通常采用金属螯合聚合物 (MCP) 标签,这些标签在灵敏度、多重性和数据采集速度方面受到限制。在这里,我们展示了纳米金属-有机框架 (NMOF) 标签,它可以同时提高 IMC 的灵敏度、多样性和采集速度。我们设计并合成了大小均匀的 Zr-NMOFs (∼31 nm,PDI < 0.1),然后用含有磷酸基团和荧光部分的异双功能化适体对其进行功能化,以生成 Zr-NMOF_Aptamer 探针。这种官能化使得与 Zr-NMOF 上的锆离子直接配体交换成为可能,从而可以同时进行荧光和质谱信号采集。荧光信号使大规模快速成像能够快速定位感兴趣区域,从而显著减少 IMC 的盲扫描时间并补偿 IMC 的较低分辨率。同时,Zr-NMOF_Aptamer 探针表现出特异性分子识别,信号放大能力比市售 MCP 探针高出四倍。此外,我们还表明 Zr-NMOF_Aptamer 探针与 IMC 分析中用于高多重共染色的市售 MCP 探针兼容。Zr-NMOF_Aptamer 探针代表了 IMC 空间蛋白质组学下一代分子探针的有前途的发展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号