当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereo- and Enantioselective Reactions of Bis(pinacolato)diboron, 1,3-Enynes, and Aldehydes Catalyzed by an Easily Accessible Bisphosphine–Cu Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-04 , DOI: 10.1021/ja5071202 Fanke Meng 1 , Fredrik Haeffner , Amir H Hoveyda
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-08-04 , DOI: 10.1021/ja5071202 Fanke Meng 1 , Fredrik Haeffner , Amir H Hoveyda
Affiliation
|
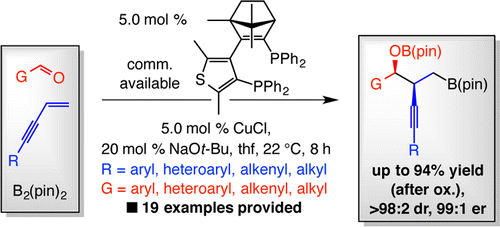
Catalytic enantioselective multicomponent processes involving bis(pinacolato)diboron [B2(pin)2], 1,3-enynes, and aldehydes are disclosed; the resulting compounds contain a primary C–B(pin) bond, as well as alkyne- and hydroxyl-substituted tertiary carbon stereogenic centers. A critical feature is the initial enantioselective Cu–B(pin) addition to an alkyne-substituted terminal alkene. This and other key mechanistic issues have been investigated by DFT calculations. Reactions are promoted by the Cu complex of a commercially available enantiomerically pure bis-phosphine and are complete in 8 h at ambient temperature; products are generated in 66–94% yield (after oxidation or catalytic cross-coupling), 90:10 to >98:2 diastereomeric ratio, and 85:15–99:1 enantiomeric ratio. Aryl-, heteroaryl-, alkenyl-, and alkyl-substituted aldehydes and enynes can be used. Utility is illustrated through catalytic alkylation and arylation of the organoboron products as well as applications to synthesis of fragments of tylonolide and mycinolide IV.
中文翻译:

易于获得的双膦-铜络合物催化双(频哪醇)二硼、1,3-烯炔和醛的非对映和对映选择性反应
公开了涉及双(频哪醇)二硼[B2(pin)2]、1,3-烯炔和醛的催化对映选择性多组分方法;所得化合物含有主 C-B(pin) 键,以及炔烃和羟基取代的叔碳立体中心。一个关键特征是初始对映选择性 Cu-B(pin) 加成到炔烃取代的末端烯烃上。这个问题和其他关键的机械问题已经通过 DFT 计算进行了研究。反应由市售对映体纯双膦的 Cu 配合物促进,并在环境温度下 8 小时内完成;产物的产率为 66–94%(氧化或催化交叉偶联后),非对映体比例为 90:10 至 >98:2,对映体比例为 85:15–99:1。可以使用芳基-、杂芳基-、烯基-和烷基-取代的醛和烯炔。通过有机硼产物的催化烷基化和芳基化以及在泰洛内酯和霉素 IV 片段的合成中的应用来说明其实用性。
更新日期:2014-08-04
中文翻译:

易于获得的双膦-铜络合物催化双(频哪醇)二硼、1,3-烯炔和醛的非对映和对映选择性反应
公开了涉及双(频哪醇)二硼[B2(pin)2]、1,3-烯炔和醛的催化对映选择性多组分方法;所得化合物含有主 C-B(pin) 键,以及炔烃和羟基取代的叔碳立体中心。一个关键特征是初始对映选择性 Cu-B(pin) 加成到炔烃取代的末端烯烃上。这个问题和其他关键的机械问题已经通过 DFT 计算进行了研究。反应由市售对映体纯双膦的 Cu 配合物促进,并在环境温度下 8 小时内完成;产物的产率为 66–94%(氧化或催化交叉偶联后),非对映体比例为 90:10 至 >98:2,对映体比例为 85:15–99:1。可以使用芳基-、杂芳基-、烯基-和烷基-取代的醛和烯炔。通过有机硼产物的催化烷基化和芳基化以及在泰洛内酯和霉素 IV 片段的合成中的应用来说明其实用性。





























 京公网安备 11010802027423号
京公网安备 11010802027423号