当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
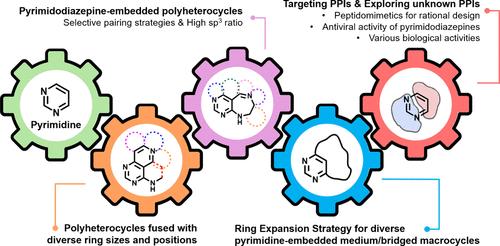
Revisiting Pyrimidine-Embedded Molecular Frameworks to Probe the Unexplored Chemical Space for Protein–Protein Interactions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-31 , DOI: 10.1021/acs.accounts.4c00452 Jeong Yeon Yoo, Yoona Choi, Heejun Kim, Seung Bum Park
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-10-31 , DOI: 10.1021/acs.accounts.4c00452 Jeong Yeon Yoo, Yoona Choi, Heejun Kim, Seung Bum Park
|
Protein–protein interactions (PPIs) are essential in numerous biological processes and diseases, making them attractive yet challenging drug targets. While many advances have been made in traditional drug discovery, targeting PPIs has been difficult due to a lack of specialized chemical libraries designed to modulate these interactions. Current libraries mainly focus on conventional target proteins like enzymes or receptors as substrate analogs rather than small-molecule modulators targeting PPIs. These traditional drug targets behave differently from PPIs. Conventional druggable targets have relatively small surfaces and binding pockets that have allowed them to be targeted with current libraries, but PPIs behave differently than these traditional drug targets. As a result, there is an urgent need for an innovative approach to expand the druggable space.
中文翻译:

重新审视嘧啶包埋的分子框架以探索蛋白质-蛋白质相互作用的未探索化学空间
蛋白质-蛋白质相互作用 (PPI) 在许多生物过程和疾病中至关重要,使其成为有吸引力但具有挑战性的药物靶标。虽然传统药物发现取得了许多进展,但由于缺乏旨在调节这些相互作用的专用化学库,靶向 PPI 一直很困难。目前的文库主要关注作为底物类似物的常规靶蛋白(如酶或受体),而不是靶向 PPI 的小分子调节剂。这些传统药物靶点的行为与 PPI 不同。传统的可成药靶点具有相对较小的表面和结合口袋,这使得它们能够被当前的文库靶向,但 PPI 的行为与这些传统药物靶点不同。因此,迫切需要一种创新方法来扩大可成药领域。
更新日期:2024-10-31
中文翻译:

重新审视嘧啶包埋的分子框架以探索蛋白质-蛋白质相互作用的未探索化学空间
蛋白质-蛋白质相互作用 (PPI) 在许多生物过程和疾病中至关重要,使其成为有吸引力但具有挑战性的药物靶标。虽然传统药物发现取得了许多进展,但由于缺乏旨在调节这些相互作用的专用化学库,靶向 PPI 一直很困难。目前的文库主要关注作为底物类似物的常规靶蛋白(如酶或受体),而不是靶向 PPI 的小分子调节剂。这些传统药物靶点的行为与 PPI 不同。传统的可成药靶点具有相对较小的表面和结合口袋,这使得它们能够被当前的文库靶向,但 PPI 的行为与这些传统药物靶点不同。因此,迫切需要一种创新方法来扩大可成药领域。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号