当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterotelechelic Organometallic PEG Reagents Enable Modular Access to Complex Bioconjugates
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-10-31 , DOI: 10.1021/acsmacrolett.4c00588 Grace E. Kunkel, Joseph W. Treacy, Magdalena F. Polite, Hayden R. Montgomery, Evan A. Doud, Kendall N. Houk, Alexander M. Spokoyny, Heather D. Maynard
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-10-31 , DOI: 10.1021/acsmacrolett.4c00588 Grace E. Kunkel, Joseph W. Treacy, Magdalena F. Polite, Hayden R. Montgomery, Evan A. Doud, Kendall N. Houk, Alexander M. Spokoyny, Heather D. Maynard
|
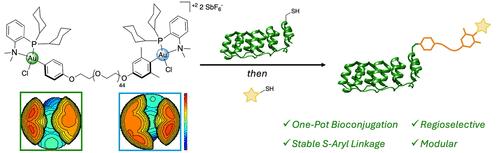
Organometallic oxidative addition complexes (OACs) have recently emerged as a powerful class of reagents for the rapid and chemoselective modification of biomolecules. Notably, the steric and electronic properties of the ligand and aryl group can be modified to tune the kinetic profile of the reaction and permit regioselective S-arylation. Using the recently developed dicyclohexylphosphine-based bidentate P,N-ligated Au(III) OACs, we computationally and experimentally examined the effects of sterically bulky and electron deficient aryl substrates to achieve selective S-arylation. With this mechanistic insight, aryl substrates based on 4-iodoanisole and 3,5-dimethyl-4-iodoanisole were incorporated as end groups to generate a heterotelechelic bis-Au(III) poly(ethylene glycol) (PEG). This reagent performed rapid and regioselective S-arylation with a model biomolecule, designed ankyrin repeat protein (DARPin), to form a protein–polymer OAC in situ. This OAC mediated a second S-arylation with biologically relevant thiolated small molecules (metal chelator, saccharide, and fluorophore) and macromolecules (polymer and therapeutic peptide). It is envisioned that this approach could be utilized for the rapid construction of biomacromolecular heteroconjugates with S-aryl linkages.
中文翻译:

异源螯合有机金属 PEG 试剂可实现对复杂生物偶联物的模块化访问
有机金属氧化加成复合物 (OAC) 最近已成为一类用于生物分子快速和化学选择性修饰的强大试剂。值得注意的是,配体和芳基的空间和电子性质可以被修饰以调整反应的动力学曲线并允许区域选择性 S-芳基化。使用最近开发的基于二环己基膦的双齿 P,N 连接的 Au(III) OAC,我们计算和实验检查了空间庞大和缺电子的芳基底物对实现选择性 S-芳基化的影响。凭借这种机制见解,基于 4-碘苯甲醚和 3,5-二甲基-4-碘苯甲醚的芳基底物作为端基掺入,以生成异螯合双金 (III) 聚乙二醇 (PEG)。该试剂使用模型生物分子进行快速和区域选择性的 S-芳基化,设计锚蛋白重复蛋白 (DARPin),原位形成蛋白质-聚合物 OAC。该 OAC 介导了具有生物学相关性巯基化小分子(金属螯合剂、糖类和荧光团)和大分子(聚合物和治疗肽)的第二次 S-芳基化。预计这种方法可用于快速构建具有 S-芳基键的生物大分子异偶联物。
更新日期:2024-10-31
中文翻译:

异源螯合有机金属 PEG 试剂可实现对复杂生物偶联物的模块化访问
有机金属氧化加成复合物 (OAC) 最近已成为一类用于生物分子快速和化学选择性修饰的强大试剂。值得注意的是,配体和芳基的空间和电子性质可以被修饰以调整反应的动力学曲线并允许区域选择性 S-芳基化。使用最近开发的基于二环己基膦的双齿 P,N 连接的 Au(III) OAC,我们计算和实验检查了空间庞大和缺电子的芳基底物对实现选择性 S-芳基化的影响。凭借这种机制见解,基于 4-碘苯甲醚和 3,5-二甲基-4-碘苯甲醚的芳基底物作为端基掺入,以生成异螯合双金 (III) 聚乙二醇 (PEG)。该试剂使用模型生物分子进行快速和区域选择性的 S-芳基化,设计锚蛋白重复蛋白 (DARPin),原位形成蛋白质-聚合物 OAC。该 OAC 介导了具有生物学相关性巯基化小分子(金属螯合剂、糖类和荧光团)和大分子(聚合物和治疗肽)的第二次 S-芳基化。预计这种方法可用于快速构建具有 S-芳基键的生物大分子异偶联物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号