当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neoadjuvant vidutolimod and nivolumab in high-risk resectable melanoma: A prospective phase II trial
Cancer Cell ( IF 48.8 ) Pub Date : 2024-10-31 , DOI: 10.1016/j.ccell.2024.10.007 Diwakar Davar, Robert M. Morrison, Amiran K. Dzutsev, Arivarasan Karunamurthy, Joe-Marc Chauvin, Florent Amatore, Julie S. Deutsch, Rodrigo X. Das Neves, Richard R. Rodrigues, John A. McCulloch, Hong Wang, Douglas J. Hartman, Jonathan H. Badger, Miriam R. Fernandes, Yulong Bai, Jie Sun, Alicia M. Cole, Poonam Aggarwal, Jennifer R. Fang, Christopher Deitrick, Riyue Bao, Umamaheswar Duvvuri, Shaum S. Sridharan, Seungwon W. Kim, Haroon A. Choudry, Matthew P. Holtzman, James F. Pingpank, James Patrick O'Toole, Richelle DeBlasio, Yang Jin, Quanquan Ding, Wentao Gao, Christopher Groetsch, Ornella Pagliano, Amy Rose, Corey Urban, Jagjit Singh, Prajan Divarkar, David Mauro, Dmitri Bobilev, James Wooldridge, Arthur M. Krieg, Matthew G. Fury, Jeffrey R. Whiteaker, Lei Zhao, Amanda G. Paulovich, Yana G. Najjar, Jason J. Luke, John M. Kirkwood, Janis M. Taube, Hyun Jung Park, Giorgio Trinchieri, Hassane M. Zarour
Cancer Cell ( IF 48.8 ) Pub Date : 2024-10-31 , DOI: 10.1016/j.ccell.2024.10.007 Diwakar Davar, Robert M. Morrison, Amiran K. Dzutsev, Arivarasan Karunamurthy, Joe-Marc Chauvin, Florent Amatore, Julie S. Deutsch, Rodrigo X. Das Neves, Richard R. Rodrigues, John A. McCulloch, Hong Wang, Douglas J. Hartman, Jonathan H. Badger, Miriam R. Fernandes, Yulong Bai, Jie Sun, Alicia M. Cole, Poonam Aggarwal, Jennifer R. Fang, Christopher Deitrick, Riyue Bao, Umamaheswar Duvvuri, Shaum S. Sridharan, Seungwon W. Kim, Haroon A. Choudry, Matthew P. Holtzman, James F. Pingpank, James Patrick O'Toole, Richelle DeBlasio, Yang Jin, Quanquan Ding, Wentao Gao, Christopher Groetsch, Ornella Pagliano, Amy Rose, Corey Urban, Jagjit Singh, Prajan Divarkar, David Mauro, Dmitri Bobilev, James Wooldridge, Arthur M. Krieg, Matthew G. Fury, Jeffrey R. Whiteaker, Lei Zhao, Amanda G. Paulovich, Yana G. Najjar, Jason J. Luke, John M. Kirkwood, Janis M. Taube, Hyun Jung Park, Giorgio Trinchieri, Hassane M. Zarour
|
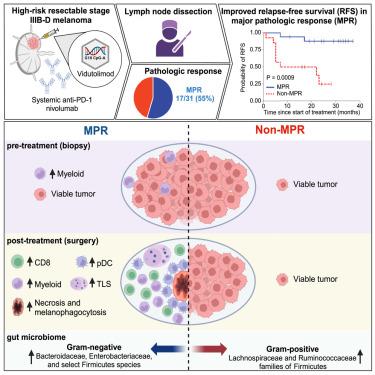
Intratumoral TLR9 agonists and anti-PD-1 produce clinical responses and broad immune activation. We conducted a single-arm study of neoadjuvant TLR9 agonist vidutolimod combined with anti-PD-1 nivolumab in high-risk resectable melanoma. In 31 evaluable patients, 55% major pathologic response (MPR) was observed, meeting primary endpoint. MPR was associated with necrosis, and melanophagocytosis with increased CD8+ tumor-infiltrating lymphocytes and plasmacytoid dendritic cells (pDCs) in the tumor microenvironment, and increased frequencies of Ki67+ CD8+ T cells peripherally. MPRs had an enriched pre-treatment gene signature of myeloid cells, and response to therapy was associated with gene signatures of immune cells, pDCs, phagocytosis, and macrophage activation. MPRs gut microbiota were enriched for Gram-negative bacteria belonging to the Bacteroidaceae and Enterobacteriaceae families and the small subgroup of Gram-negative Firmicutes. Our findings support that combined vidutolimod and nivolumab stimulates a broad anti-tumor immune response and is associated with distinct baseline myeloid gene signature and gut microbiota. ClinicalTrials.gov identifier: NCT03618641 .
中文翻译:

新辅助 vidutolimod 和 nivolumab 治疗高危可切除黑色素瘤:一项前瞻性 II 期试验
瘤内 TLR9 激动剂和抗 PD-1 产生临床反应和广泛的免疫激活。我们进行了一项新辅助 TLR9 激动剂 vidutolimod 联合抗 PD-1 纳武利尤单抗治疗高危可切除黑色素瘤的单臂研究。在 31 例可评估的患者中,观察到 55% 的主要病理反应 (MPR),达到主要终点。MPR 与坏死相关,黑素吞噬作用与肿瘤微环境中 CD8 + 肿瘤浸润淋巴细胞和浆细胞样树突状细胞 (pDC) 增加有关,以及外周 Ki67 + CD8 + T 细胞频率增加。MPRs 具有丰富的骨髓细胞治疗前基因特征,对治疗的反应与免疫细胞、 pDC 、吞噬作用和巨噬细胞活化的基因特征相关。MPRs 肠道菌群中富含属于拟杆菌科和肠杆菌科的革兰氏阴性菌以及革兰氏阴性菌门的小亚群。我们的研究结果支持 vidutolimod 和 nivolumab 联合治疗可刺激广泛的抗肿瘤免疫反应,并且与不同的基线髓系基因特征和肠道微生物群相关。ClinicalTrials.gov 标识符:NCT03618641。
更新日期:2024-10-31
中文翻译:

新辅助 vidutolimod 和 nivolumab 治疗高危可切除黑色素瘤:一项前瞻性 II 期试验
瘤内 TLR9 激动剂和抗 PD-1 产生临床反应和广泛的免疫激活。我们进行了一项新辅助 TLR9 激动剂 vidutolimod 联合抗 PD-1 纳武利尤单抗治疗高危可切除黑色素瘤的单臂研究。在 31 例可评估的患者中,观察到 55% 的主要病理反应 (MPR),达到主要终点。MPR 与坏死相关,黑素吞噬作用与肿瘤微环境中 CD8 + 肿瘤浸润淋巴细胞和浆细胞样树突状细胞 (pDC) 增加有关,以及外周 Ki67 + CD8 + T 细胞频率增加。MPRs 具有丰富的骨髓细胞治疗前基因特征,对治疗的反应与免疫细胞、 pDC 、吞噬作用和巨噬细胞活化的基因特征相关。MPRs 肠道菌群中富含属于拟杆菌科和肠杆菌科的革兰氏阴性菌以及革兰氏阴性菌门的小亚群。我们的研究结果支持 vidutolimod 和 nivolumab 联合治疗可刺激广泛的抗肿瘤免疫反应,并且与不同的基线髓系基因特征和肠道微生物群相关。ClinicalTrials.gov 标识符:NCT03618641。






























 京公网安备 11010802027423号
京公网安备 11010802027423号