Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-ligand redox in layered oxide cathodes for Li-ion batteries
Joule ( IF 38.6 ) Pub Date : 2024-10-30 , DOI: 10.1016/j.joule.2024.10.007 Matthew J.W. Ogley, Ashok S. Menon, Gaurav C. Pandey, Galo J. Páez Fajardo, Beth J. Johnston, Innes McClelland, Veronika Majherova, Steven Huband, Debashis Tripathy, Israel Temprano, Stefano Agrestini, Veronica Celorrio, Gabriel E. Pérez, Samuel G. Booth, Clare P. Grey, Serena A. Cussen, Louis F.J. Piper
Joule ( IF 38.6 ) Pub Date : 2024-10-30 , DOI: 10.1016/j.joule.2024.10.007 Matthew J.W. Ogley, Ashok S. Menon, Gaurav C. Pandey, Galo J. Páez Fajardo, Beth J. Johnston, Innes McClelland, Veronika Majherova, Steven Huband, Debashis Tripathy, Israel Temprano, Stefano Agrestini, Veronica Celorrio, Gabriel E. Pérez, Samuel G. Booth, Clare P. Grey, Serena A. Cussen, Louis F.J. Piper
|
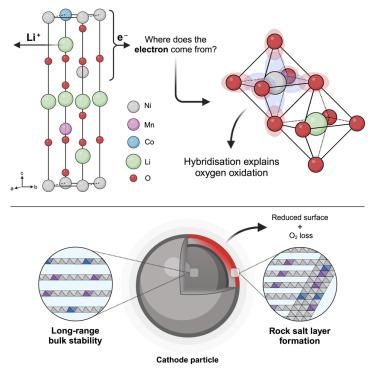
This study refutes the commonly used ionic-bonding model that demarcates transition metal (TM) and oxygen redox using an archetypal Ni-rich layered oxide cathode, LiNi0.8Mn0.1Co0.1O2. Here, charge compensation during delithiation occurs without formal (ionic) Ni oxidation. Instead, oxygen-dominated states control the redox process, facilitated by strong TM-O hybridization, forming bulk-stable 3d8L and 3d8L2 electronic states, where L is a ligand hole. Bulk O–O dimers are observed with O K-edge resonant inelastic X-ray scattering but, critically, without the long-range TM migration or void formation observed in Li-rich layered oxides. Above 4.34 V vs. Li+/Li, the cathode loses O, forming a resistive surface rock-salt layer that causes capacity fade. This highlights the importance of cathode engineering when attempting to achieve higher energy densities with layered oxide cathodes, especially in those where O dominates the charge compensation mechanism.
中文翻译:

用于锂离子电池的层状氧化物阴极中的金属配体氧化还原
本研究驳斥了常用的离子键合模型,该模型使用典型的富镍层状氧化物阴极 LiNi0.8Mn0.1Co0.1O2 来划分过渡金属 (TM) 和氧氧化还原。在这里,脱锂过程中的电荷补偿发生在没有正式(离子)Ni 氧化的情况下。相反,以氧为主的状态控制氧化还原过程,由强 TM-O 杂化促进,形成体积稳定的 3d8L 和 3d8L2 电子态,其中 L 是配体空穴。用 O K 边共振非弹性 X 射线散射观察到本体 O-O 二聚体,但关键是,没有在富锂层状氧化物中观察到的长程 TM 迁移或空隙形成。相对于 Li+/Li,高于 4.34 V,阴极失去 O,形成电阻表面岩盐层,导致容量衰减。这凸显了阴极工程在尝试使用层状氧化物阴极实现更高的能量密度时的重要性,尤其是在 O 主导电荷补偿机制的阴极中。
更新日期:2024-10-30
中文翻译:

用于锂离子电池的层状氧化物阴极中的金属配体氧化还原
本研究驳斥了常用的离子键合模型,该模型使用典型的富镍层状氧化物阴极 LiNi0.8Mn0.1Co0.1O2 来划分过渡金属 (TM) 和氧氧化还原。在这里,脱锂过程中的电荷补偿发生在没有正式(离子)Ni 氧化的情况下。相反,以氧为主的状态控制氧化还原过程,由强 TM-O 杂化促进,形成体积稳定的 3d8L 和 3d8L2 电子态,其中 L 是配体空穴。用 O K 边共振非弹性 X 射线散射观察到本体 O-O 二聚体,但关键是,没有在富锂层状氧化物中观察到的长程 TM 迁移或空隙形成。相对于 Li+/Li,高于 4.34 V,阴极失去 O,形成电阻表面岩盐层,导致容量衰减。这凸显了阴极工程在尝试使用层状氧化物阴极实现更高的能量密度时的重要性,尤其是在 O 主导电荷补偿机制的阴极中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号