Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ovarian cancer-derived IL-4 promotes immunotherapy resistance
Cell ( IF 45.5 ) Pub Date : 2024-10-30 , DOI: 10.1016/j.cell.2024.10.006 Gurkan Mollaoglu, Alexander Tepper, Chiara Falcomatà, Hunter T. Potak, Luisanna Pia, Angelo Amabile, Jaime Mateus-Tique, Noam Rabinovich, Matthew D. Park, Nelson M. LaMarche, Rachel Brody, Lindsay Browning, Jia-Ren Lin, Dmitriy Zamarin, Peter K. Sorger, Sandro Santagata, Miriam Merad, Alessia Baccarini, Brian D. Brown
Cell ( IF 45.5 ) Pub Date : 2024-10-30 , DOI: 10.1016/j.cell.2024.10.006 Gurkan Mollaoglu, Alexander Tepper, Chiara Falcomatà, Hunter T. Potak, Luisanna Pia, Angelo Amabile, Jaime Mateus-Tique, Noam Rabinovich, Matthew D. Park, Nelson M. LaMarche, Rachel Brody, Lindsay Browning, Jia-Ren Lin, Dmitriy Zamarin, Peter K. Sorger, Sandro Santagata, Miriam Merad, Alessia Baccarini, Brian D. Brown
|
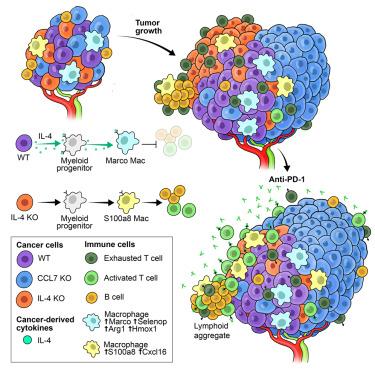
Ovarian cancer is resistant to immunotherapy, and this is influenced by the immunosuppressed tumor microenvironment (TME) dominated by macrophages. Resistance is also affected by intratumoral heterogeneity, whose development is poorly understood. To identify regulators of ovarian cancer immunity, we employed a spatial functional genomics screen (Perturb-map), focused on receptor/ligands hypothesized to be involved in tumor-macrophage communication. Perturb-map recapitulated tumor heterogeneity and revealed that interleukin-4 (IL-4) promotes resistance to anti-PD-1. We find ovarian cancer cells are the key source of IL-4, which directs the formation of an immunosuppressive TME via macrophage control. IL-4 loss was not compensated by nearby IL-4-expressing clones, revealing short-range regulation of TME composition dictating tumor evolution. Our studies show heterogeneous TMEs can emerge from localized altered expression of cancer-derived cytokines/chemokines that establish immune-rich and immune-excluded neighborhoods, which drive clone selection and immunotherapy resistance. They also demonstrate the potential of targeting IL-4 signaling to enhance ovarian cancer response to immunotherapy.
中文翻译:

卵巢癌来源的 IL-4 促进免疫治疗耐药
卵巢癌对免疫治疗具有耐药性,这受到以巨噬细胞为主的免疫抑制肿瘤微环境 (TME) 的影响。耐药性也受到瘤内异质性的影响,其发展知之甚少。为了确定卵巢癌免疫的调节因子,我们采用了空间功能基因组学筛选 (Perturb-map),重点关注假设参与肿瘤-巨噬细胞通讯的受体/配体。Perturb-map 概括了肿瘤异质性,并揭示了白细胞介素 4 (IL-4) 促进对抗 PD-1 的耐药性。我们发现卵巢癌细胞是 IL-4 的关键来源,IL-4 通过巨噬细胞控制指导免疫抑制性 TME 的形成。IL-4 丢失不能被附近表达 IL-4 的克隆补偿,揭示了 TME 组成的短程调节决定了肿瘤的演变。我们的研究表明,异质性 TME 可以从癌症衍生的细胞因子/趋化因子的局部改变表达中出现,这些细胞因子/趋化因子建立了免疫丰富和免疫排除的邻域,从而驱动克隆选择和免疫治疗耐药性。它们还展示了靶向 IL-4 信号传导以增强卵巢癌对免疫治疗反应的潜力。
更新日期:2024-10-30
中文翻译:

卵巢癌来源的 IL-4 促进免疫治疗耐药
卵巢癌对免疫治疗具有耐药性,这受到以巨噬细胞为主的免疫抑制肿瘤微环境 (TME) 的影响。耐药性也受到瘤内异质性的影响,其发展知之甚少。为了确定卵巢癌免疫的调节因子,我们采用了空间功能基因组学筛选 (Perturb-map),重点关注假设参与肿瘤-巨噬细胞通讯的受体/配体。Perturb-map 概括了肿瘤异质性,并揭示了白细胞介素 4 (IL-4) 促进对抗 PD-1 的耐药性。我们发现卵巢癌细胞是 IL-4 的关键来源,IL-4 通过巨噬细胞控制指导免疫抑制性 TME 的形成。IL-4 丢失不能被附近表达 IL-4 的克隆补偿,揭示了 TME 组成的短程调节决定了肿瘤的演变。我们的研究表明,异质性 TME 可以从癌症衍生的细胞因子/趋化因子的局部改变表达中出现,这些细胞因子/趋化因子建立了免疫丰富和免疫排除的邻域,从而驱动克隆选择和免疫治疗耐药性。它们还展示了靶向 IL-4 信号传导以增强卵巢癌对免疫治疗反应的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号