当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
π-Electron-Assisted Charge Storage in Fused-Ring Aromatic Carbonyl Electrodes for Aqueous Manganese-Ion Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-10-29 , DOI: 10.1021/acsenergylett.4c02418 Hyungjin Lee, Amey Nimkar, Netanel Shpigel, Daniel Sharon, Seung-Tae Hong, Doron Aurbach, Munseok S. Chae
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-10-29 , DOI: 10.1021/acsenergylett.4c02418 Hyungjin Lee, Amey Nimkar, Netanel Shpigel, Daniel Sharon, Seung-Tae Hong, Doron Aurbach, Munseok S. Chae
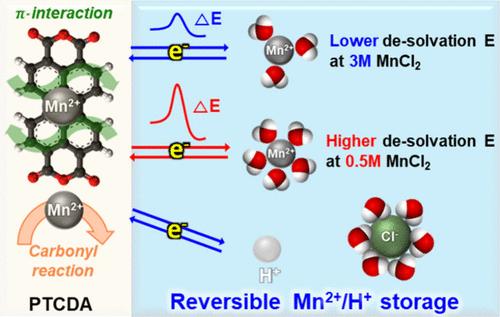
|
Rechargeable manganese batteries hold promise for large-scale energy storage due to the abundance and eco-friendly nature of manganese. A key challenge is developing cathode materials capable of reversibly inserting Mn ions with a high specific capacity. Here, we demonstrate that perylene-3,4,9,10-tetracarboxylic dianhydride electrodes efficiently and reversibly insert Mn2+ ions in 3 M MnCl2 aqueous electrolyte solutions. Leveraging the carbonyl groups and the π-electron configuration, such compounds can serve as robust redox centers, facilitating reversible interactions with divalent ions such as Mn2+. Through comprehensive studies involving electrochemistry, elemental analyses, spectroscopy, and structural analysis, we explored these systems and found them as promising anode materials for Mn batteries. Demonstrating excellent Mn storage capabilities, such molecules could attain a reversible capacity of approximately >185 mAh g–1 at a current density of 100 mA g–1, maintaining an average voltage of approximately 0.8 V vs Mn/Mn2+, while exhibiting notable capacity retention.
中文翻译:

用于水系锰离子电池的熔融环芳香族羰基电极中的 π-电子辅助电荷存储
由于锰的丰富性和环保性,可充电锰电池有望实现大规模储能。一个关键挑战是开发能够可逆插入具有高比容量的 Mn 离子的正极材料。在这里,我们证明了苝-3,4,9,10-四羧酸二酐电极可有效且可逆地将 Mn2+ 离子插入 3 M MnCl2 电解质水溶液中。利用羰基和π电子构型,这些化合物可以用作稳健的氧化还原中心,促进与 Mn2+ 等二价离子的可逆相互作用。通过涉及电化学、元素分析、光谱学和结构分析的综合研究,我们探索了这些系统,发现它们是有前途的 Mn 电池负极材料。这种分子表现出优异的 Mn 存储能力,在 100 mA g–1 的电流密度下可以达到大约 >185 mAh g–1 的可逆容量,保持约 0.8 V vs Mn/Mn2+,同时表现出显着的容量保持。
更新日期:2024-10-29
中文翻译:

用于水系锰离子电池的熔融环芳香族羰基电极中的 π-电子辅助电荷存储
由于锰的丰富性和环保性,可充电锰电池有望实现大规模储能。一个关键挑战是开发能够可逆插入具有高比容量的 Mn 离子的正极材料。在这里,我们证明了苝-3,4,9,10-四羧酸二酐电极可有效且可逆地将 Mn2+ 离子插入 3 M MnCl2 电解质水溶液中。利用羰基和π电子构型,这些化合物可以用作稳健的氧化还原中心,促进与 Mn2+ 等二价离子的可逆相互作用。通过涉及电化学、元素分析、光谱学和结构分析的综合研究,我们探索了这些系统,发现它们是有前途的 Mn 电池负极材料。这种分子表现出优异的 Mn 存储能力,在 100 mA g–1 的电流密度下可以达到大约 >185 mAh g–1 的可逆容量,保持约 0.8 V vs Mn/Mn2+,同时表现出显着的容量保持。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号