当前位置:
X-MOL 学术
›
Eur. J. Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vericiguat Global Study in Participants with Chronic Heart Failure: Design of the VICTOR trial
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-30 , DOI: 10.1002/ejhf.3501 Yogesh N.V. Reddy, Javed Butler, Kevin J. Anstrom, Robert O. Blaustein, Marc P. Bonaca, Stefano Corda, Justin A. Ezekowitz, Carolyn S.P. Lam, Eldrin F. Lewis, JoAnn Lindenfeld, Ciaran J. McMullan, Robert J. Mentz, Christopher O'Connor, Mahesh Patel, Piotr Ponikowski, Giuseppe M.C. Rosano, Clara I. Saldarriaga, Michele Senni, James Udelson, Adriaan A. Voors, Faiez Zannad
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-30 , DOI: 10.1002/ejhf.3501 Yogesh N.V. Reddy, Javed Butler, Kevin J. Anstrom, Robert O. Blaustein, Marc P. Bonaca, Stefano Corda, Justin A. Ezekowitz, Carolyn S.P. Lam, Eldrin F. Lewis, JoAnn Lindenfeld, Ciaran J. McMullan, Robert J. Mentz, Christopher O'Connor, Mahesh Patel, Piotr Ponikowski, Giuseppe M.C. Rosano, Clara I. Saldarriaga, Michele Senni, James Udelson, Adriaan A. Voors, Faiez Zannad
|
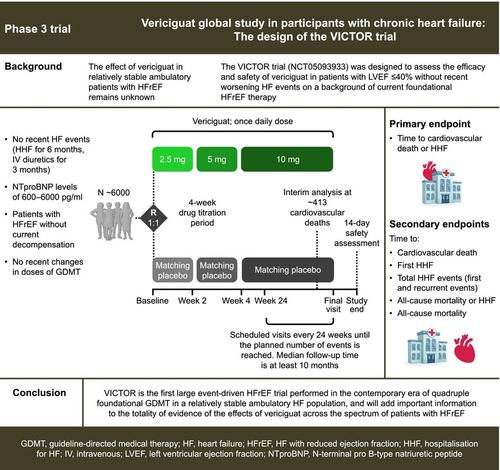
AimsIn the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial, the soluble guanylate cyclase stimulator vericiguat reduced the risk of hospitalization for heart failure (HHF) or cardiovascular death in patients with heart failure (HF) and reduced ejection fraction (HFrEF) with recent worsening HF. The effect of vericiguat in patients with HFrEF without recent worsening HF remains unknown. The VICTOR (Vericiguat Global Study in Participants with Chronic Heart Failure) trial was designed to assess the efficacy and safety of vericiguat in patients with ejection fraction ≤40% without recent worsening HF on a background of current foundational HFrEF therapy.MethodsThe primary endpoint for VICTOR is time to first event for the composite of HHF or cardiovascular death. The trial will also assess the effect of vericiguat on time to cardiovascular death, time to HHF, total HHF, and all‐cause death. As an event‐driven trial, at least 1080 primary events are expected, but follow‐up will continue until the targeted number of at least 590 cardiovascular deaths has been reached. Approximately 6000 participants will be randomized to vericiguat or placebo.ConclusionVICTOR is the first large event‐driven HFrEF trial performed in the contemporary era of quadruple foundational guideline‐directed medical therapy, in a compensated ambulatory HF population. VICTOR will add important information to the evidence of the effects of vericiguat across the spectrum of patients with HFrEF.
中文翻译:

慢性心力衰竭参与者的 Vericiguat 全球研究:VICTOR 试验的设计
目的在 VICTORIA(射血分数降低的心力衰竭受试者的 Vericiguat 全球研究)试验中,可溶性鸟苷酸环化酶刺激剂 vericiguat 降低了心力衰竭 (HF) 和射血分数降低 (HFrEF) 患者因心力衰竭 (HHF) 或心血管死亡住院的风险,最近 HF 恶化。vericiguat 对近期 HF 无恶化的 HFrEF 患者的影响仍然未知。VICTOR (Vericiguat 慢性心力衰竭参与者全球研究) 试验旨在评估 vericiguat 在射血分数 ≤40% 且近期 HF 没有恶化的患者中的疗效和安全性,背景是当前基础 HFrEF 治疗。方法VICTOR 的主要终点是 HHF 或心血管死亡复合的首次事件发生时间。该试验还将评估 vericiguat 对心血管死亡时间、HHF 时间、总 HHF 和全因死亡的影响。作为一项事件驱动试验,预计至少有 1080 个原发事件,但随访将继续进行,直到达到至少 590 例心血管死亡的目标数量。大约 6000 名参与者将被随机分配到 vericiguat 或安慰剂组。结论VICTOR 是当代第一个大型事件驱动的 HFrEF 试验,在代偿性非卧床 HF 人群中进行四重基础指南指导的药物治疗。VICTOR 将为 vericiguat 对 HFrEF 患者影响的证据增加重要信息。
更新日期:2024-10-30
中文翻译:

慢性心力衰竭参与者的 Vericiguat 全球研究:VICTOR 试验的设计
目的在 VICTORIA(射血分数降低的心力衰竭受试者的 Vericiguat 全球研究)试验中,可溶性鸟苷酸环化酶刺激剂 vericiguat 降低了心力衰竭 (HF) 和射血分数降低 (HFrEF) 患者因心力衰竭 (HHF) 或心血管死亡住院的风险,最近 HF 恶化。vericiguat 对近期 HF 无恶化的 HFrEF 患者的影响仍然未知。VICTOR (Vericiguat 慢性心力衰竭参与者全球研究) 试验旨在评估 vericiguat 在射血分数 ≤40% 且近期 HF 没有恶化的患者中的疗效和安全性,背景是当前基础 HFrEF 治疗。方法VICTOR 的主要终点是 HHF 或心血管死亡复合的首次事件发生时间。该试验还将评估 vericiguat 对心血管死亡时间、HHF 时间、总 HHF 和全因死亡的影响。作为一项事件驱动试验,预计至少有 1080 个原发事件,但随访将继续进行,直到达到至少 590 例心血管死亡的目标数量。大约 6000 名参与者将被随机分配到 vericiguat 或安慰剂组。结论VICTOR 是当代第一个大型事件驱动的 HFrEF 试验,在代偿性非卧床 HF 人群中进行四重基础指南指导的药物治疗。VICTOR 将为 vericiguat 对 HFrEF 患者影响的证据增加重要信息。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号