当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable, aromatic, and electrophilic azepinium ions: Design using quantum chemical methods
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-10-30 , DOI: 10.1002/jcc.27520 Nabajyoti Patra, Astha Gupta, Prasad V. Bharatam
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-10-30 , DOI: 10.1002/jcc.27520 Nabajyoti Patra, Astha Gupta, Prasad V. Bharatam
|
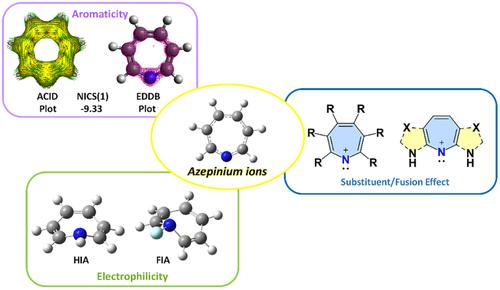
Cyclic nitrenium ions containing five-membered and six-membered rings are available, however, the seven-membered cyclic nitrenium ions (azepinium ions) are rare. The chemistry of these species is related to their stability originating from the aromaticity due to 6π electrons. Very few theoretical and experimental studies have been conducted on the azepinium ions. Related clozapine and olanzapine cations (diazepinium ions) were observed during drug metabolism studies. In this work, quantum chemical analysis has been carried out to estimate the stability, aromaticity, and electrophilicity of several derivatives of azepinium ions. A few of the designed azepinium ions carry ΔES-T values in the range of 50 kcal/mol favoring singlet state; π donating groups at the 2nd position increase the singlet-triplet energy differences. Most of the substituents reduce the NICS(1) values compared to the parent system. Ring fusion with heterocyclic five-membered rings generally increases the aromaticity and the stability of the azepinium ion ring systems. The electrophilicity parameters estimated in terms of HIA, FIA, and ω values indicate that it is possible to fine-tune the chemical properties of azepinium ions with appropriate modulation.
中文翻译:

稳定、芳香族和亲电氮杂离子:使用量子化学方法设计
含有五元和六元环的环状氮离子是可用的,但是,七元环状氮离子(氮杂离子)很少见。这些物质的化学性质与它们的稳定性有关,这种稳定性源于由 6π 电子引起的芳香性。对氮杂磷离子进行的理论和实验研究很少。在药物代谢研究中观察到相关的氯氮平和奥氮平阳离子 (二氮平离子)。在这项工作中,已经进行了量子化学分析以估计氮杂平离子的几种衍生物的稳定性、芳香性和亲电性。一些设计的氮杂离子携带的 ΔES-T 值在 50 kcal/mol 范围内,有利于单重态;π 第 2 位的供体组增加了单重态-三重态能量差。与母系统相比,大多数取代基降低了 NICS(1) 值。与杂环五元环的环熔合通常会增加氮杂鎓离子环系统的芳香性和稳定性。以 HIA、FIA 和 ω 值估计的亲电性参数表明,可以通过适当的调制来微调氮杂离子的化学性质。
更新日期:2024-10-30
中文翻译:

稳定、芳香族和亲电氮杂离子:使用量子化学方法设计
含有五元和六元环的环状氮离子是可用的,但是,七元环状氮离子(氮杂离子)很少见。这些物质的化学性质与它们的稳定性有关,这种稳定性源于由 6π 电子引起的芳香性。对氮杂磷离子进行的理论和实验研究很少。在药物代谢研究中观察到相关的氯氮平和奥氮平阳离子 (二氮平离子)。在这项工作中,已经进行了量子化学分析以估计氮杂平离子的几种衍生物的稳定性、芳香性和亲电性。一些设计的氮杂离子携带的 ΔES-T 值在 50 kcal/mol 范围内,有利于单重态;π 第 2 位的供体组增加了单重态-三重态能量差。与母系统相比,大多数取代基降低了 NICS(1) 值。与杂环五元环的环熔合通常会增加氮杂鎓离子环系统的芳香性和稳定性。以 HIA、FIA 和 ω 值估计的亲电性参数表明,可以通过适当的调制来微调氮杂离子的化学性质。































 京公网安备 11010802027423号
京公网安备 11010802027423号