当前位置:
X-MOL 学术
›
Coord. Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the nature’s discriminating factors behind the selection of molybdoenzymes and tungstoenzymes depending on the biological environment
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.ccr.2024.216290 Udita Das, Ankita Das, Asim K. Das
Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.ccr.2024.216290 Udita Das, Ankita Das, Asim K. Das
|
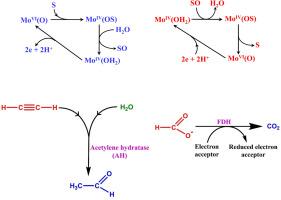
Pterin based molybdenum cofactor found in the molybdenum dependent enzymes catalyzes the oxo-transferase and hydroxylase activity. For tungsten, the pterin based tungsten cofactor is known for the similar biological low potential redox activities in anaerobic conditions in thermophilic microorganisms. Nature's selection of tungsten and molybdenum for the different working conditions is rationalized in terms of their relative bioavailabilities, thermodynamic stabilities of their compounds, kinetic inertness and the difference in relativistic effect experienced by these two congeners. The relativistic effect is the most important factor to justify the tungsten vs. molybdenum selectivity in different enzymes. The non-innocent dithiolene based pterin ligand tunes the biological redox activity of the enzymes by stabilising +4, +5 and +6 oxidation states of molybdenum and tungsten. In fact, it acts as a ‘redox buffer’ in their catalytic mechanism. Mechanistic aspects of the enzymatic activity are more investigated for the Mo-dependent enzymes compared to those of W-dependent enzymes. Strong controversies regarding the mechanisms of activity of the enzymes like Mo/W-FDH (formate dehydrogenase), Mo-Cu-CODH (carbon monoxide dehydrogenase), W-AOR (aldehyde oxidoreductase), W-AH (acetylene hydratase), W-BCRs (benzoyl-CoA-reductases), etc. , indicate that this field is still an active area of research.
中文翻译:

探索根据生物环境选择钼酶和钨酶背后的自然界鉴别因素
在钼依赖性酶中发现的基于蝶呤的钼辅因子催化氧代转移酶和羟化酶活性。对于钨,基于 pterin 的钨辅因子在嗜热微生物的厌氧条件下具有相似的生物低电位氧化还原活性。大自然为不同的工作条件选择钨和钼,根据它们的相对生物利用度、它们化合物的热力学稳定性、动力学惰性以及这两个同系物所经历的相对论效应的不同来合理化。相对论效应是证明钨与钼在不同酶中的选择性的最重要因素。非无害的二硫烯基蝶呤配体通过稳定钼和钨的 +4、+5 和 +6 氧化态来调节酶的生物氧化还原活性。事实上,它在他们的催化机制中充当“氧化还原缓冲剂”。与 W 依赖性酶相比,Mo 依赖性酶的酶活性的机制方面得到了更多的研究。关于 Mo/W-FDH(甲酸盐脱氢酶)、Mo-Cu-CODH(一氧化碳脱氢酶)、W-AOR(醛氧化还原酶)、W-AH(乙炔水合酶)、W-BCR(苯甲酰辅酶 A 还原酶)等酶的活性机制存在激烈争议,表明该领域仍然是一个活跃的研究领域。
更新日期:2024-10-29
中文翻译:

探索根据生物环境选择钼酶和钨酶背后的自然界鉴别因素
在钼依赖性酶中发现的基于蝶呤的钼辅因子催化氧代转移酶和羟化酶活性。对于钨,基于 pterin 的钨辅因子在嗜热微生物的厌氧条件下具有相似的生物低电位氧化还原活性。大自然为不同的工作条件选择钨和钼,根据它们的相对生物利用度、它们化合物的热力学稳定性、动力学惰性以及这两个同系物所经历的相对论效应的不同来合理化。相对论效应是证明钨与钼在不同酶中的选择性的最重要因素。非无害的二硫烯基蝶呤配体通过稳定钼和钨的 +4、+5 和 +6 氧化态来调节酶的生物氧化还原活性。事实上,它在他们的催化机制中充当“氧化还原缓冲剂”。与 W 依赖性酶相比,Mo 依赖性酶的酶活性的机制方面得到了更多的研究。关于 Mo/W-FDH(甲酸盐脱氢酶)、Mo-Cu-CODH(一氧化碳脱氢酶)、W-AOR(醛氧化还原酶)、W-AH(乙炔水合酶)、W-BCR(苯甲酰辅酶 A 还原酶)等酶的活性机制存在激烈争议,表明该领域仍然是一个活跃的研究领域。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号