当前位置:
X-MOL 学术
›
Aliment. Pharm. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of the Effectiveness and Safety of Mesenchymal Stem Cell Treatment in Fistulising Crohn's Disease: An International Real-Life Retrospective Multicentre Cohort Study
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-10-28 , DOI: 10.1111/apt.18359 Péter Bacsur, Daniel Shaham, Zuzana Serclova, Tamás Resál, Bernadett Farkas, Patrícia Sarlós, Pál Miheller, Nitsan Maharshak, Meir Zemel, Ariella Bar-Gil Shitrit, Shlomo Yellinek, Anita Bálint, Anna Fábián, Renáta Bor, Zsófia Bősze, Emese Ivány, Zoltán Szepes, Klaudia Farkas, Illés Tóth, György Lázár, Katerina Vlkova, Aneta Tremerova, Petra Zuskova, Szabolcs Ábrahám, Tamás Molnár
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-10-28 , DOI: 10.1111/apt.18359 Péter Bacsur, Daniel Shaham, Zuzana Serclova, Tamás Resál, Bernadett Farkas, Patrícia Sarlós, Pál Miheller, Nitsan Maharshak, Meir Zemel, Ariella Bar-Gil Shitrit, Shlomo Yellinek, Anita Bálint, Anna Fábián, Renáta Bor, Zsófia Bősze, Emese Ivány, Zoltán Szepes, Klaudia Farkas, Illés Tóth, György Lázár, Katerina Vlkova, Aneta Tremerova, Petra Zuskova, Szabolcs Ábrahám, Tamás Molnár
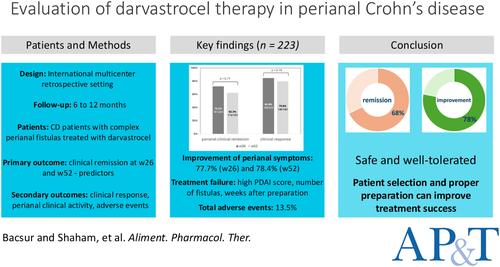
|
Perianal fistulas of Crohn's disease (CD) create a significant burden on patients' lives. However, the efficacy and safety of adipose-derived mesenchymal stem cell treatment are contradicting, and real-world evidence is lacking.
中文翻译:

间充质干细胞治疗瘘管性克罗恩病的有效性和安全性评价:一项国际现实生活中的回顾性多中心队列研究
克罗恩病 (CD) 的肛周瘘对患者的生活造成了沉重的负担。然而,脂肪来源的间充质干细胞治疗的有效性和安全性是相互矛盾的,并且缺乏真实世界的证据。
更新日期:2024-10-28
中文翻译:

间充质干细胞治疗瘘管性克罗恩病的有效性和安全性评价:一项国际现实生活中的回顾性多中心队列研究
克罗恩病 (CD) 的肛周瘘对患者的生活造成了沉重的负担。然而,脂肪来源的间充质干细胞治疗的有效性和安全性是相互矛盾的,并且缺乏真实世界的证据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号