当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
m6A/YTHDF2-mediated mRNA decay targets TGF-β signaling to suppress the quiescence acquisition of early postnatal mouse hippocampal NSCs
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.stem.2024.10.002 Feng Zhang, Yao Fu, Dennisse Jimenez-Cyrus, Ting Zhao, Yachen Shen, Yusha Sun, Zhijian Zhang, Qing Wang, Riki Kawaguchi, Daniel H. Geschwind, Chuan He, Guo-li Ming, Hongjun Song
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-10-29 , DOI: 10.1016/j.stem.2024.10.002 Feng Zhang, Yao Fu, Dennisse Jimenez-Cyrus, Ting Zhao, Yachen Shen, Yusha Sun, Zhijian Zhang, Qing Wang, Riki Kawaguchi, Daniel H. Geschwind, Chuan He, Guo-li Ming, Hongjun Song
|
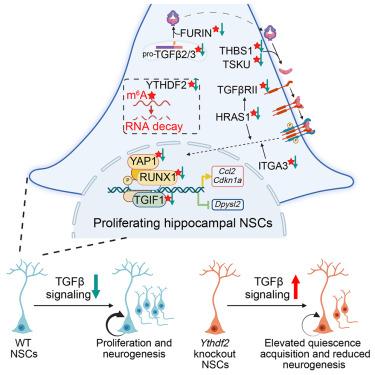
Quiescence acquisition of proliferating neural stem cells (NSCs) is required to establish the adult NSC pool. The underlying molecular mechanisms are not well understood. Here, we showed that conditional deletion of the m6A reader Ythdf2, which promotes mRNA decay, in proliferating NSCs in the early postnatal mouse hippocampus elevated quiescence acquisition in a cell-autonomous fashion with decreased neurogenesis. Multimodal profiling of m6A modification, YTHDF2 binding, and mRNA decay in hippocampal NSCs identified shared targets in multiple transforming growth factor β (TGF-β)-signaling-pathway components, including TGF-β ligands, maturation factors, receptors, transcription regulators, and signaling regulators. Functionally, Ythdf2 deletion led to TGF-β-signaling activation in NSCs, suppression of which rescued elevated quiescence acquisition of proliferating hippocampal NSCs. Our study reveals the dynamic nature and critical roles of mRNA decay in establishing the quiescent adult hippocampal NSC pool and uncovers a distinct mode of epitranscriptomic control via co-regulation of multiple components of the same signaling pathway.
中文翻译:

m6A/YTHDF2 介导的 mRNA 衰变靶向 TGF-β 信号传导,以抑制出生后早期小鼠海马 NSC 的静止获得
建立成体 NSC 库需要静止获取增殖神经干细胞 (NSC)。潜在的分子机制尚不清楚。在这里,我们表明,在出生后早期小鼠海马体增殖的 NSC 中,促进 mRNA 衰变的 m6A 阅读器 Ythdf2 的条件性缺失以细胞自主方式提高了静止获得,神经发生减少。海马 NSC 中 m6A 修饰、YTHDF2 结合和 mRNA 衰变的多模式分析确定了多个转化生长因子 β (TGF-β) 信号通路成分的共同靶标,包括 TGF β配体、成熟因子、受体、转录调节因子和信号调节因子。在功能上,Ythdf2 缺失导致 NSC 中的 TGF β信号激活,抑制其可挽救增殖海马 NSC 升高的静止获得。我们的研究揭示了 mRNA 衰变在建立静止成人海马 NSC 库中的动态性质和关键作用,并通过共同调节同一信号通路的多个组分揭示了一种独特的表观转录组控制模式。
更新日期:2024-10-29
中文翻译:

m6A/YTHDF2 介导的 mRNA 衰变靶向 TGF-β 信号传导,以抑制出生后早期小鼠海马 NSC 的静止获得
建立成体 NSC 库需要静止获取增殖神经干细胞 (NSC)。潜在的分子机制尚不清楚。在这里,我们表明,在出生后早期小鼠海马体增殖的 NSC 中,促进 mRNA 衰变的 m6A 阅读器 Ythdf2 的条件性缺失以细胞自主方式提高了静止获得,神经发生减少。海马 NSC 中 m6A 修饰、YTHDF2 结合和 mRNA 衰变的多模式分析确定了多个转化生长因子 β (TGF-β) 信号通路成分的共同靶标,包括 TGF β配体、成熟因子、受体、转录调节因子和信号调节因子。在功能上,Ythdf2 缺失导致 NSC 中的 TGF β信号激活,抑制其可挽救增殖海马 NSC 升高的静止获得。我们的研究揭示了 mRNA 衰变在建立静止成人海马 NSC 库中的动态性质和关键作用,并通过共同调节同一信号通路的多个组分揭示了一种独特的表观转录组控制模式。































 京公网安备 11010802027423号
京公网安备 11010802027423号